The Food and Drug Administration (FDA) on Friday scheduled public meeting to review COVID-19 vaccines for the youngest children in the U.S.
company said the shot was about 51% effective against the virus for kids under 2 years old and about 37% effective in kids ages 2 to 5.
It added that similar requests are underway with international regulatory authorities, noting that the requests are based on a 25-microgram two-dose primary series of the vaccine. "We are proud to share that we have initiated our EUA submission for authorization for our COVID-19 vaccine for young children," Moderna CEO Stéphane Bancel said in a statement."We believe mRNA-1273 will be able to safely protect these children against SARS-CoV-2, which is so important in our continued fight against COVID-19 and will be especially welcomed by parents and caregivers.
Right now, only children ages 5 or older can be vaccinated in the U.S., using rival Pfizer’s vaccine.Pfizer is also expected to announce if three of its even smaller-dose shots work for the youngest children. Right now, only children ages 5 or older can be vaccinated in the U.S., using rival Pfizer’s vaccine.Julia Musto is a reporter for Fox News Digital. You can find her on Twitter at @JuliaElenaMusto.
日本 最新ニュース, 日本 見出し
Similar News:他のニュース ソースから収集した、これに似たニュース記事を読むこともできます。
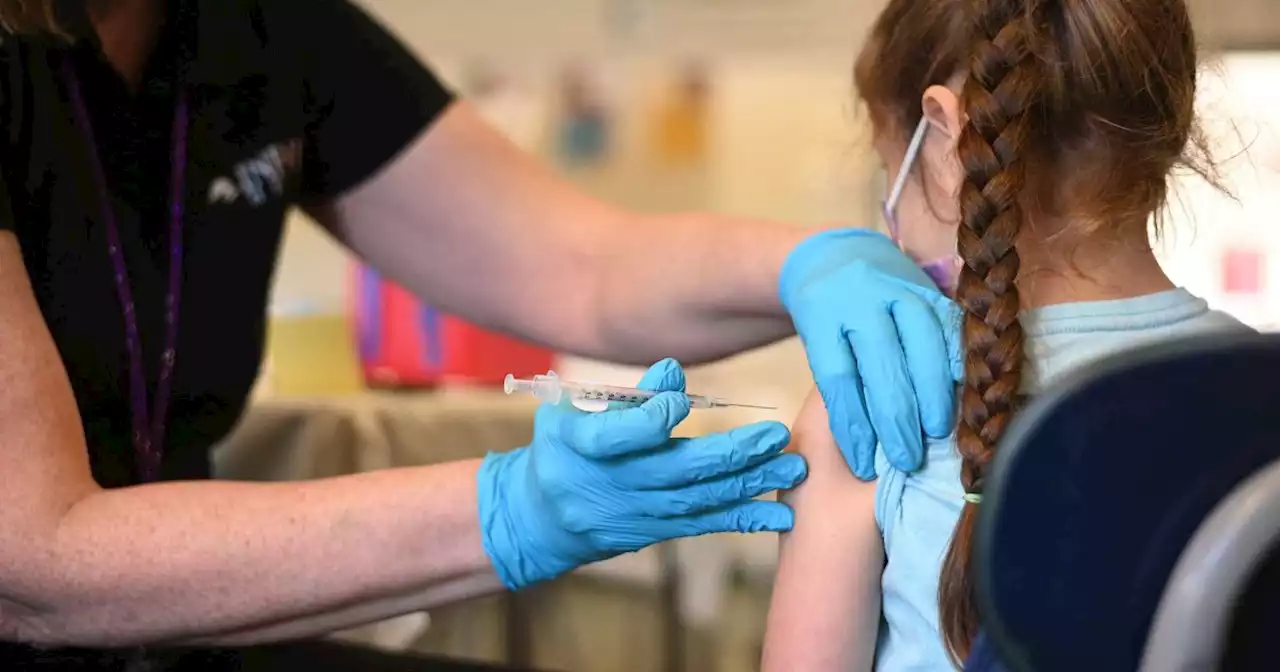 FDA sets June dates to meet on COVID vaccine for young kidsThe agency’s advisory committee has been asked to convene to make recommendations on Moderna and Pfizer’s vaccines for the youngest age group.
FDA sets June dates to meet on COVID vaccine for young kidsThe agency’s advisory committee has been asked to convene to make recommendations on Moderna and Pfizer’s vaccines for the youngest age group.
続きを読む »
 FDA Sets June Meetings on COVID Vaccines for Youngest KidsThe meeting announcement follows months of frustration from families impatient for a chance to vaccinate their little children, along with complaints from politicians bemoaning the slow pace of the process.
FDA Sets June Meetings on COVID Vaccines for Youngest KidsThe meeting announcement follows months of frustration from families impatient for a chance to vaccinate their little children, along with complaints from politicians bemoaning the slow pace of the process.
続きを読む »
 FDA to review COVID vaccines for youngest kids in JuneThe agency set tentative dates in June to publicly review the vaccines, typically the final step before authorizing the shots.
FDA to review COVID vaccines for youngest kids in JuneThe agency set tentative dates in June to publicly review the vaccines, typically the final step before authorizing the shots.
続きを読む »
 FDA sets June meetings on COVID vaccines for youngest kidsThe FDA said it plans to convene its outside panel of vaccine experts on June 8, 21 and 22 to review applications from Moderna and Pfizer for child vaccines.
FDA sets June meetings on COVID vaccines for youngest kidsThe FDA said it plans to convene its outside panel of vaccine experts on June 8, 21 and 22 to review applications from Moderna and Pfizer for child vaccines.
続きを読む »
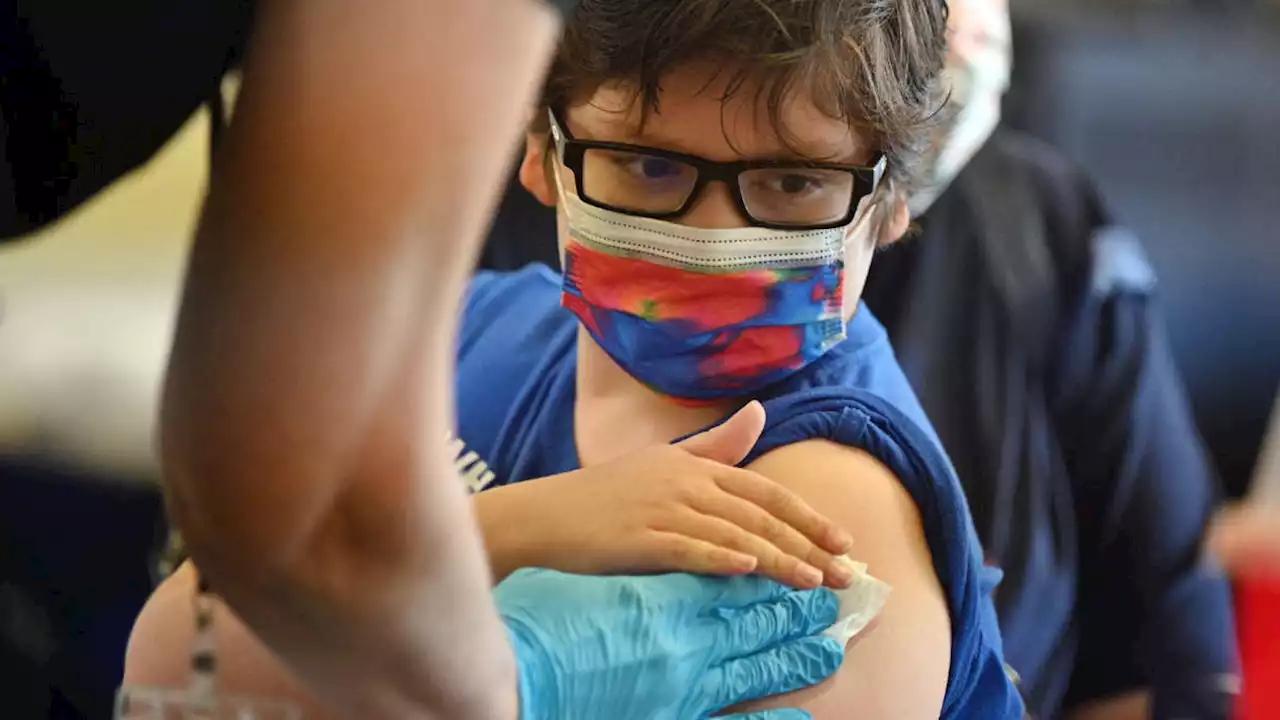 COVID Vaccines for Kids Under 5 Could be Available in June, FDA Official SaysAn FDA official has said that coronavirus vaccines for children under the age of 5 will be considered — and likely approved — as soon as June.
COVID Vaccines for Kids Under 5 Could be Available in June, FDA Official SaysAn FDA official has said that coronavirus vaccines for children under the age of 5 will be considered — and likely approved — as soon as June.
続きを読む »
 FDA could authorize COVID vaccine for children 5 and under, and Novavax vaccine, this JuneThe Food and Drug Administration released a tentative schedule of meetings for its outside vaccine advisers in June on vaccines for the youngest children.
FDA could authorize COVID vaccine for children 5 and under, and Novavax vaccine, this JuneThe Food and Drug Administration released a tentative schedule of meetings for its outside vaccine advisers in June on vaccines for the youngest children.
続きを読む »
