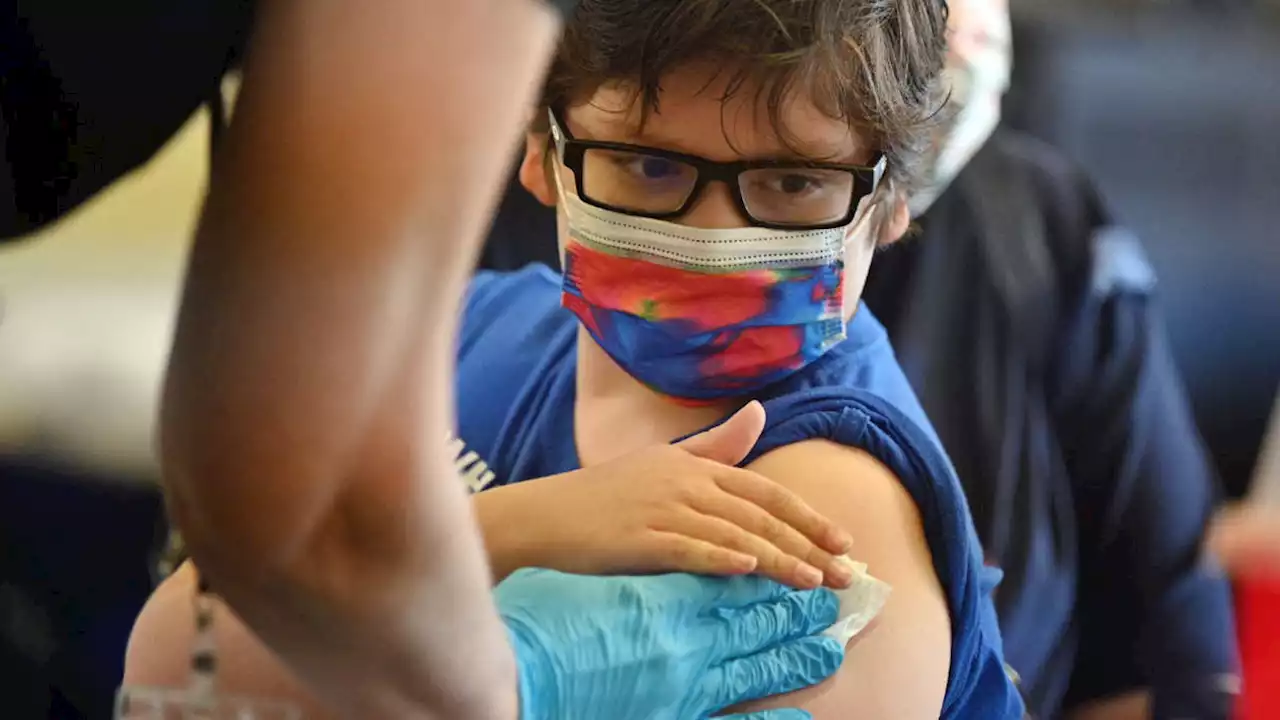
An FDA official has said that coronavirus vaccines for children under the age of 5 will be considered — and likely approved — as soon as June.
“We are not going to delay things unnecessarily here. This whole concept of delaying is not something we will be doing,”Once the agency has met with its advisers, the vaccines could be approved within the same month, Marks added, stating that the agency “would anticipate June authorizations for one or more of the pediatric vaccinations.”
While vaccines for children that are 5 years old and older have been granted emergency use authorization,, as mask requirements have been relaxed in many places, leaving those without vaccines vulnerable to infection. Concern for younger kids is warranted. The mortality rate for children under 5 is much lower than it is for others, but not nonexistent — indeed, according to Centers for Disease Control and Prevention statistics,Many children have also suffered from long-term effects after contracting coronavirus, a condition that is commonly known as “long COVID.
Experts also warn that there is not enough research on long COVID in children to determine just how widespread it is, though in the United Kingdom, it’s estimated that
日本 最新ニュース, 日本 見出し
Similar News:他のニュース ソースから収集した、これに似たニュース記事を読むこともできます。
 FDA Sets June Meetings on COVID Vaccines for Youngest KidsThe meeting announcement follows months of frustration from families impatient for a chance to vaccinate their little children, along with complaints from politicians bemoaning the slow pace of the process.
FDA Sets June Meetings on COVID Vaccines for Youngest KidsThe meeting announcement follows months of frustration from families impatient for a chance to vaccinate their little children, along with complaints from politicians bemoaning the slow pace of the process.
続きを読む »
 FDA to review COVID vaccines for youngest kids in JuneThe agency set tentative dates in June to publicly review the vaccines, typically the final step before authorizing the shots.
FDA to review COVID vaccines for youngest kids in JuneThe agency set tentative dates in June to publicly review the vaccines, typically the final step before authorizing the shots.
続きを読む »
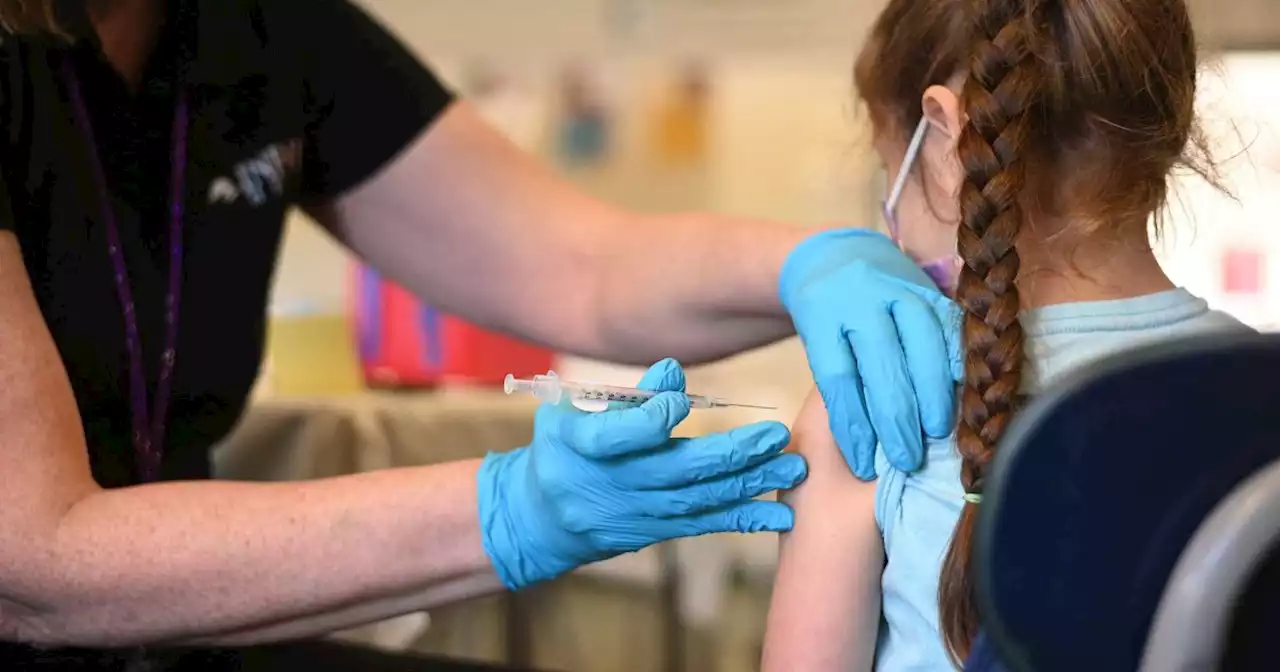 FDA sets June dates to meet on COVID vaccine for young kidsThe agency’s advisory committee has been asked to convene to make recommendations on Moderna and Pfizer’s vaccines for the youngest age group.
FDA sets June dates to meet on COVID vaccine for young kidsThe agency’s advisory committee has been asked to convene to make recommendations on Moderna and Pfizer’s vaccines for the youngest age group.
続きを読む »
 FDA sets June meetings on COVID vaccines for youngest kidsThe FDA said it plans to convene its outside panel of vaccine experts on June 8, 21 and 22 to review applications from Moderna and Pfizer for child vaccines.
FDA sets June meetings on COVID vaccines for youngest kidsThe FDA said it plans to convene its outside panel of vaccine experts on June 8, 21 and 22 to review applications from Moderna and Pfizer for child vaccines.
続きを読む »
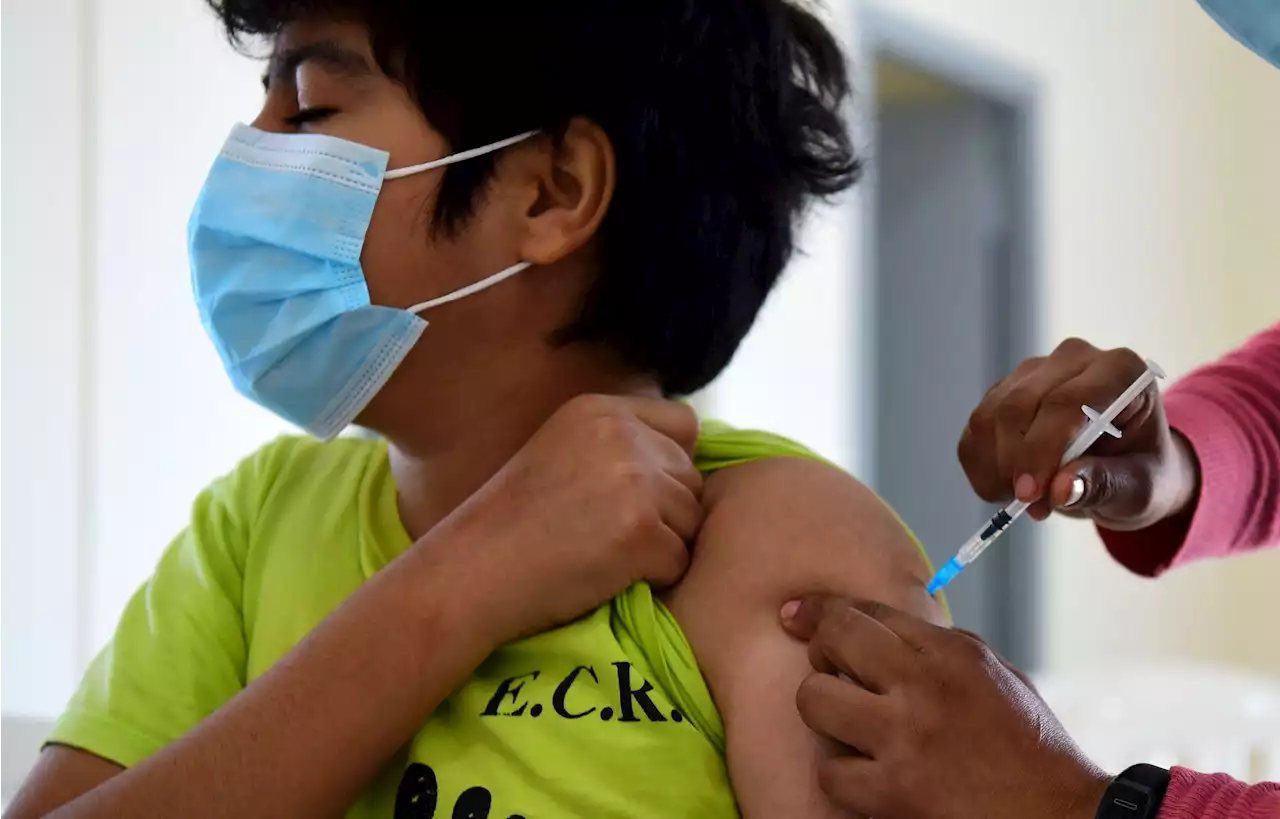 Pfizer Asks FDA To Authorize Covid Booster For Kids 5-11As the omicron variant puts children at greater risk of hospitalization, public health officials have worked to make Covid-19 treatments more accessible to young people.
Pfizer Asks FDA To Authorize Covid Booster For Kids 5-11As the omicron variant puts children at greater risk of hospitalization, public health officials have worked to make Covid-19 treatments more accessible to young people.
続きを読む »
 Moderna to ask FDA to authorize Covid-19 vaccine for young kidsThe request comes a month after the drug company signaled its two-dose regimen generated immune protection in the youngest children comparable to young adults.
Moderna to ask FDA to authorize Covid-19 vaccine for young kidsThe request comes a month after the drug company signaled its two-dose regimen generated immune protection in the youngest children comparable to young adults.
続きを読む »