General Mills and other packaged-food giants say the government's push to tighten food labeling rules is unconstitutional.
As the federal government moves to change rules for what foods can sport"healthy" labels, manufacturers are pushing back.
That's unfair, many food companies gripe, complaining that the more rigorous nutritional standards would wrongly malign a range of popular foods. The rule"automatically disqualifies entire categories of nutrient dense foods," Kellogg's wrote in a February 16 comment on the agency's proposal. The window for submitting public comments on the proposed rules closed earlier this month. The FDA will now review the feedback, though the timing of a final rule is uncertain.The Consumer Brands Association, whose members include Coca-Cola, PepsiCo, Hain Celestial and the Campbell Soup Company, took a similar tack, writing that"consumers have a First Amendment right to receive truthful information about products and manufacturers have a First Amendment right to provide it to them.
"Consumers already know that whole fruits and vegetables are healthy and may not be consuming such products for a variety of reasons," the Institute said, adding that the FDA should be"meeting people where they are." "We want to use policies that advocate for a healthier diet than we currently have, and that's why we believe the healthy claims should be allowed only for foods that are truly healthy," said Eva Greenthal, senior policy scientist at the Center for Science in the Public Interest. The CSPI mostly supports the new rules but says they allow too much refined grain and processed fruit.
Greenthal sees the revised FDA guidelines as mostly positive, although she doubts it will have much impact on Americans' eating habits.
日本 最新ニュース, 日本 見出し
Similar News:他のニュース ソースから収集した、これに似たニュース記事を読むこともできます。
 Washington state attorney general says FDA rules on abortion drug are unreasonableA coalition of state attorneys general is suing the Food and Drug Administration over its regulation of mifepristone. NPR's Michel Martin speaks with Washington state Attorney General Bob Ferguson.
Washington state attorney general says FDA rules on abortion drug are unreasonableA coalition of state attorneys general is suing the Food and Drug Administration over its regulation of mifepristone. NPR's Michel Martin speaks with Washington state Attorney General Bob Ferguson.
続きを読む »
 PolitiFact - FDA approval is required for COVID-19 vaccine manufacturers to change ingredients in shotsThe COVID-19 vaccines from Pfizer and Moderna are no longer under an emergency use authorization for adults. Even if they were, any changes would require the manufacturers to submit data and have their product reassessed by the FDA.
PolitiFact - FDA approval is required for COVID-19 vaccine manufacturers to change ingredients in shotsThe COVID-19 vaccines from Pfizer and Moderna are no longer under an emergency use authorization for adults. Even if they were, any changes would require the manufacturers to submit data and have their product reassessed by the FDA.
続きを読む »
 First at-home combination test for COVID and flu authorized by FDAThe FDA has authorized the first over-the-counter at-home test that can detect and differentiate between a test result for flu and one for COVID-19.
First at-home combination test for COVID and flu authorized by FDAThe FDA has authorized the first over-the-counter at-home test that can detect and differentiate between a test result for flu and one for COVID-19.
続きを読む »
 FDA authorizes combination flu-COVID test for home useWhile at-home COVID tests are readily available, this is the first home test for influenza A and B, commonly known as the flu.
FDA authorizes combination flu-COVID test for home useWhile at-home COVID tests are readily available, this is the first home test for influenza A and B, commonly known as the flu.
続きを読む »
 FDA authorizes first at-home joint flu, COVID-19 testThe Food and Drug Administration has issued an emergency use authorization for the first over-the-counter at-home test than can detect whether someone has the flu or COVID-19.
FDA authorizes first at-home joint flu, COVID-19 testThe Food and Drug Administration has issued an emergency use authorization for the first over-the-counter at-home test than can detect whether someone has the flu or COVID-19.
続きを読む »
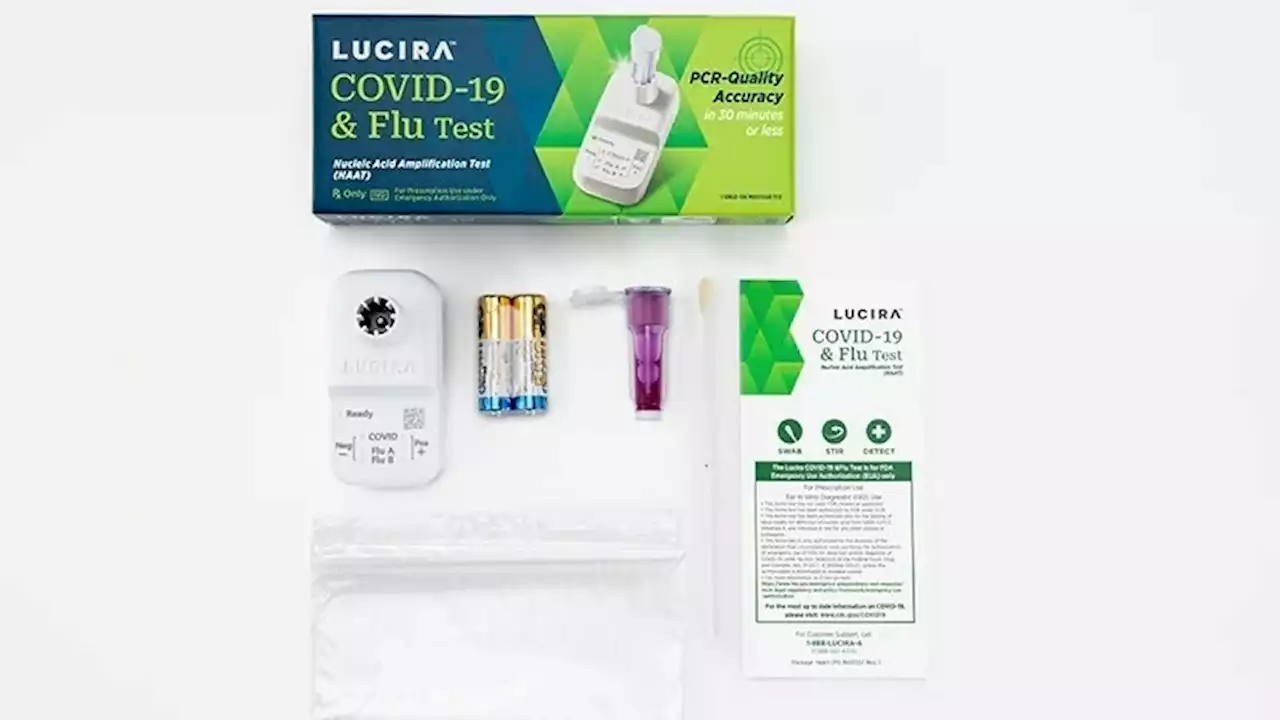 FDA authorizes the first at-home test for COVID-19 and the fluThe FDA has authorized the first at-home test that can simultaneously detect both COVID and the flu. The nasal swab provides results within 30 minutes.
FDA authorizes the first at-home test for COVID-19 and the fluThe FDA has authorized the first at-home test that can simultaneously detect both COVID and the flu. The nasal swab provides results within 30 minutes.
続きを読む »
