The Food and Drug Administration has issued a warning concerning WanaBana Apple Cinnamon Fruit Puree Pouches.
The FDA has announced the recall of WanaBana apple cinnamon fruit puree after several children tested positive for high levels of lead in their blood.All pouches of the apple cinnamon pouches have been recalled. They had been sold at several stores including Sam’s Club, Amazon and Dollar Tree.
If your child has eaten the recalled puree, you’re being directed to call your child’s doctor to get a blood test, theThe recall was prompted by an investigation by the North Carolina Department of Health and Human Services and the North Carolina Department of Agriculture & Consumer Services after four children had high lead levels in their blood. The NCDHHS determined they were exposed to the lead from the WanaBana apple cinnamon fruit puree.
日本 最新ニュース, 日本 見出し
Similar News:他のニュース ソースから収集した、これに似たニュース記事を読むこともできます。
 FDA urging people to be cautious after lead found in WanaBana Brand Apple Cinnamon PureeThe FDA released an advisory Saturday warning consumers after elevated levels of lead were found in WanaBana apple cinnamon fruit puree pouches.
FDA urging people to be cautious after lead found in WanaBana Brand Apple Cinnamon PureeThe FDA released an advisory Saturday warning consumers after elevated levels of lead were found in WanaBana apple cinnamon fruit puree pouches.
続きを読む »
 FDA urging people to be cautious after lead found in WanaBana Brand Apple Cinnamon PureeThe FDA released an advisory Saturday warning consumers after elevated levels of lead were found in WanaBana apple cinnamon fruit puree pouches.
FDA urging people to be cautious after lead found in WanaBana Brand Apple Cinnamon PureeThe FDA released an advisory Saturday warning consumers after elevated levels of lead were found in WanaBana apple cinnamon fruit puree pouches.
続きを読む »
 The FDA warns consumers to stop using several eyedrop products due to infection riskFederal health officials issued the alert for six different brands of products after finding bacterial contamination at a manufacturing facility.
The FDA warns consumers to stop using several eyedrop products due to infection riskFederal health officials issued the alert for six different brands of products after finding bacterial contamination at a manufacturing facility.
続きを読む »
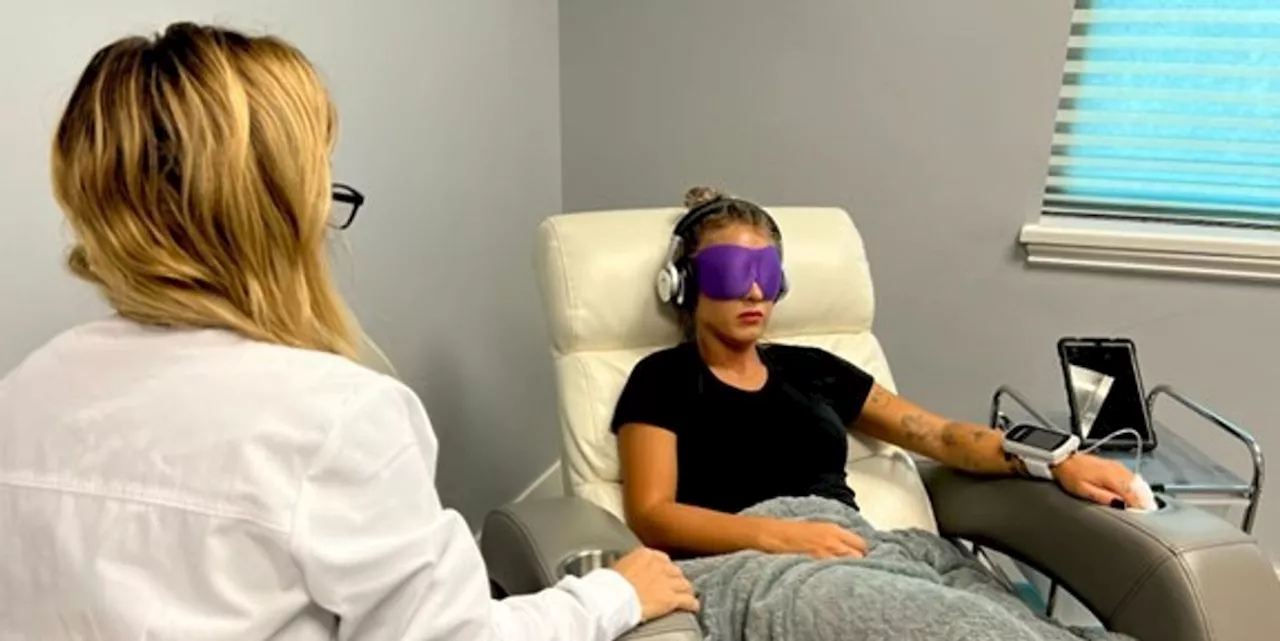 Venture Firms Push for FDA Approval of Psychedelic Drugs for VeteransSkeptics question the merits of giving psychoactive drugs to veterans following the opioid crisis
Venture Firms Push for FDA Approval of Psychedelic Drugs for VeteransSkeptics question the merits of giving psychoactive drugs to veterans following the opioid crisis
続きを読む »
 FDA issues warning to stop using several eyedrop products due to infection riskThe products were sold at CVS, Rite Aid, Target and other stores and pharmacies.
FDA issues warning to stop using several eyedrop products due to infection riskThe products were sold at CVS, Rite Aid, Target and other stores and pharmacies.
続きを読む »
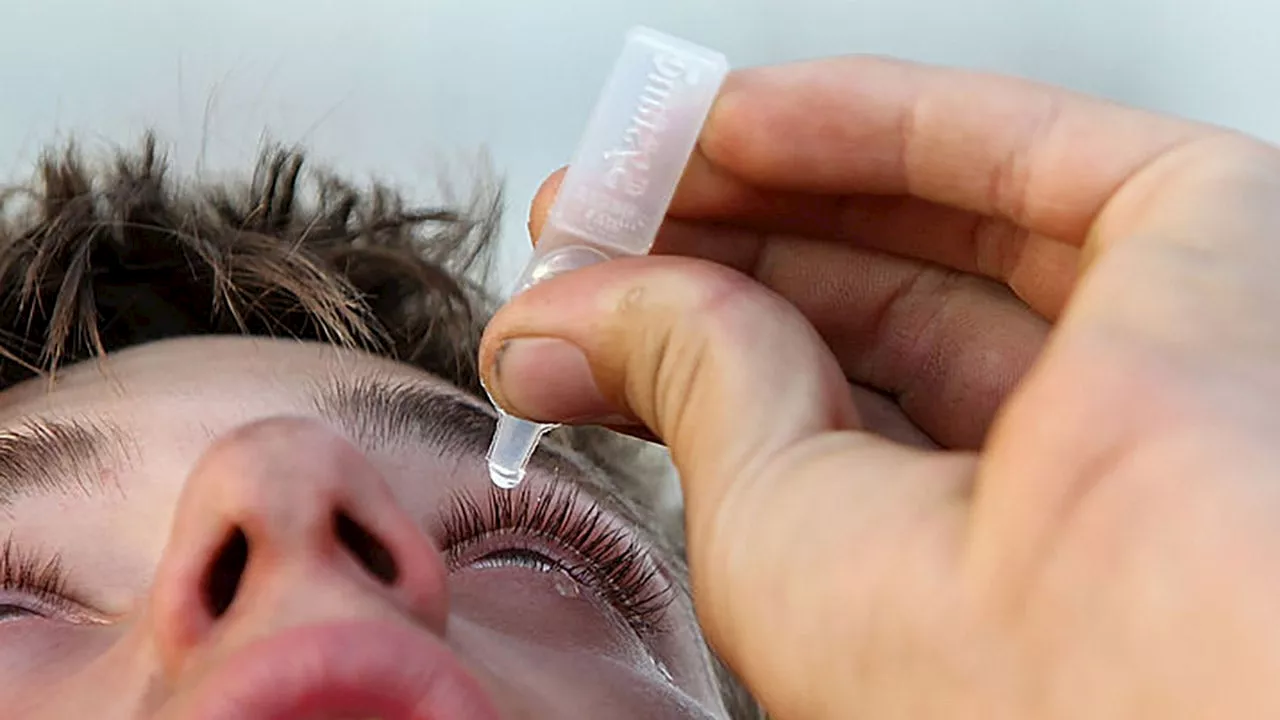 FDA issues warning over several eyedrop products due to infection riskConsumers should stop using 26 over-the-counter eye drop products over the potential risk of eye infections that could lead to vision loss, the FDA said.
FDA issues warning over several eyedrop products due to infection riskConsumers should stop using 26 over-the-counter eye drop products over the potential risk of eye infections that could lead to vision loss, the FDA said.
続きを読む »
