Americans may soon get a new COVID-19 vaccine option — a more traditional kind of shot known as a protein vaccine.
The company behind a COVID-19 vaccine will miss a target for giving doses to the U.N.-backed effort to deliver shots to poorer countries.
Erck said the Serum Institute recently passed an FDA inspection, clearing the way for the agency to finish evaluating the vaccine.Earlier in the pandemic, large studies in the U.S., Mexico and Britain found two doses of the Novavax vaccine were safe and about 90% effective at preventing symptomatic COVID-19. When the Delta variant emerged last summer, Novavax reported a booster dose revved up virus-fighting antibodies that could tackle that strain.
日本 最新ニュース, 日本 見出し
Similar News:他のニュース ソースから収集した、これに似たニュース記事を読むこともできます。
 Novavax hopes its COVID shot wins over FDA, vaccine holdoutsGAITHERSBURG, Md. (AP) — Americans may soon get a new COVID-19 vaccine option -- shots made with a more tried-and-true technology than today’s versions. The big question: Why should they care? After long delays, the Food and Drug Administration is expected to decide within weeks whether to authorize Novavax's vaccine.
Novavax hopes its COVID shot wins over FDA, vaccine holdoutsGAITHERSBURG, Md. (AP) — Americans may soon get a new COVID-19 vaccine option -- shots made with a more tried-and-true technology than today’s versions. The big question: Why should they care? After long delays, the Food and Drug Administration is expected to decide within weeks whether to authorize Novavax's vaccine.
続きを読む »
 Novavax hopes its COVID shot wins over FDA, vaccine holdoutsAmericans may soon get a new COVID-19 vaccine option — a more traditional kind of shot known as a protein vaccine.
Novavax hopes its COVID shot wins over FDA, vaccine holdoutsAmericans may soon get a new COVID-19 vaccine option — a more traditional kind of shot known as a protein vaccine.
続きを読む »
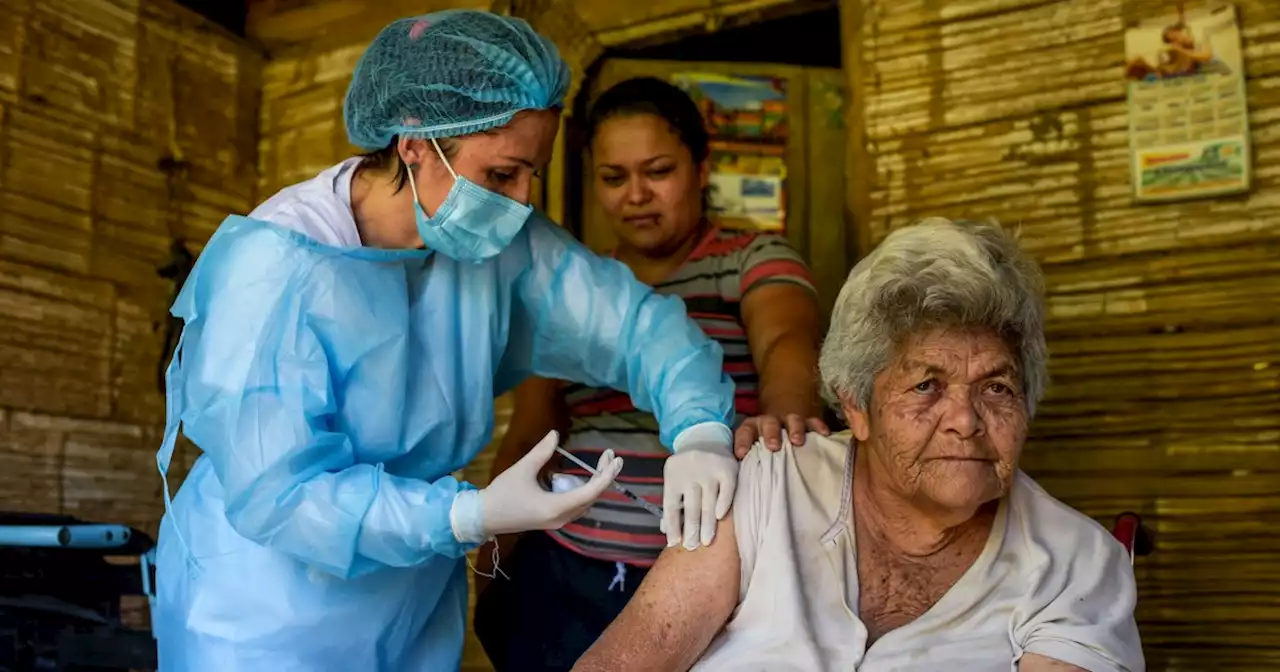 Can Novavax win over vaccine holdouts with its Covid shot?The late entrant into the U.S. vaccination effort uses the same technology seen in vaccines for hepatitis B and shingles, among others.
Can Novavax win over vaccine holdouts with its Covid shot?The late entrant into the U.S. vaccination effort uses the same technology seen in vaccines for hepatitis B and shingles, among others.
続きを読む »
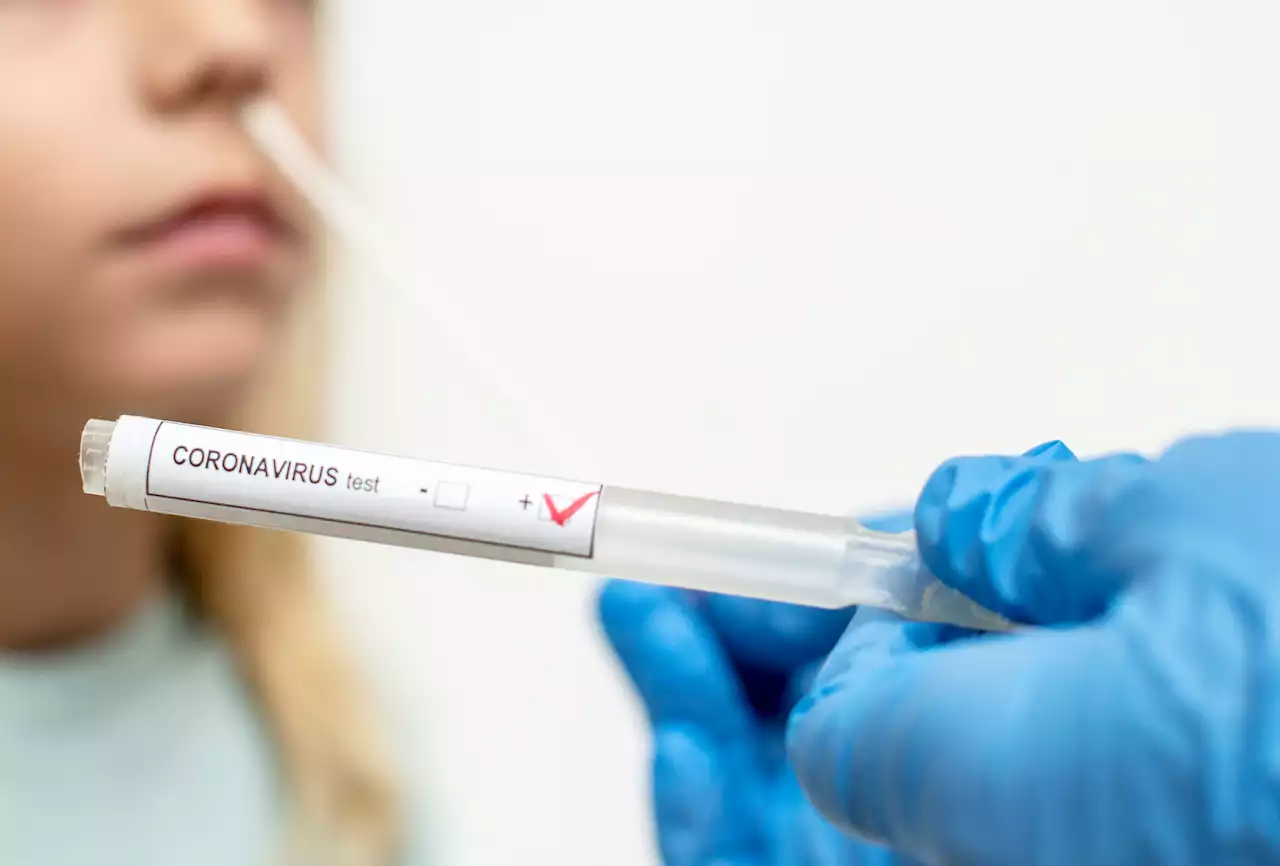 How Long Are You Protected After Getting COVID and Can You Get COVID Twice?For those who test positive for COVID and experience symptoms, how long could they last?
How Long Are You Protected After Getting COVID and Can You Get COVID Twice?For those who test positive for COVID and experience symptoms, how long could they last?
続きを読む »
 Pfizer again asks FDA to authorize Covid vaccine for youngest kidsNEW: Pfizer-BioNTech says it has asked the FDA to authorize its Covid-19 vaccine in children ages 6 months to 4 years — the only group that remains ineligible for vaccination.
Pfizer again asks FDA to authorize Covid vaccine for youngest kidsNEW: Pfizer-BioNTech says it has asked the FDA to authorize its Covid-19 vaccine in children ages 6 months to 4 years — the only group that remains ineligible for vaccination.
続きを読む »
