Narcan nasal spray is on the fast track to be approved as an over-the-country emergency overdose treatment, especially for fentanyl.
When Narcan nasal spray is used while someone is overdosing on opioids it can reverse the frequently fatal symptoms. Right now, federal regulators are looking to approve Narcan sales over the counter in pharmacies in an effort to curb the carnage, largely from fentanyl.& CEO, said.
Five years ago there were thousands of overdoses citywide, averaging 18 per day; from the ballparks to Navy Pier and the Magnificent Mile.With the increased misuse, and mistaken use, of fentanyl, deadly overdoses across Illinois continue to climb. The U.S. Food and Drug Administration is now moving to make Narcan more easily available. Regulators are giving priority review of the over-the-counter application by Emergent BioSolutions for its nasal spray Narcan, also known by the active drug name naloxone.
Quick FDA over the counter approval would give parents, roommates, and friends of opioid users the easy ability to have Narcan spray on hand in case of an overdose.
日本 最新ニュース, 日本 見出し
Similar News:他のニュース ソースから収集した、これに似たニュース記事を読むこともできます。
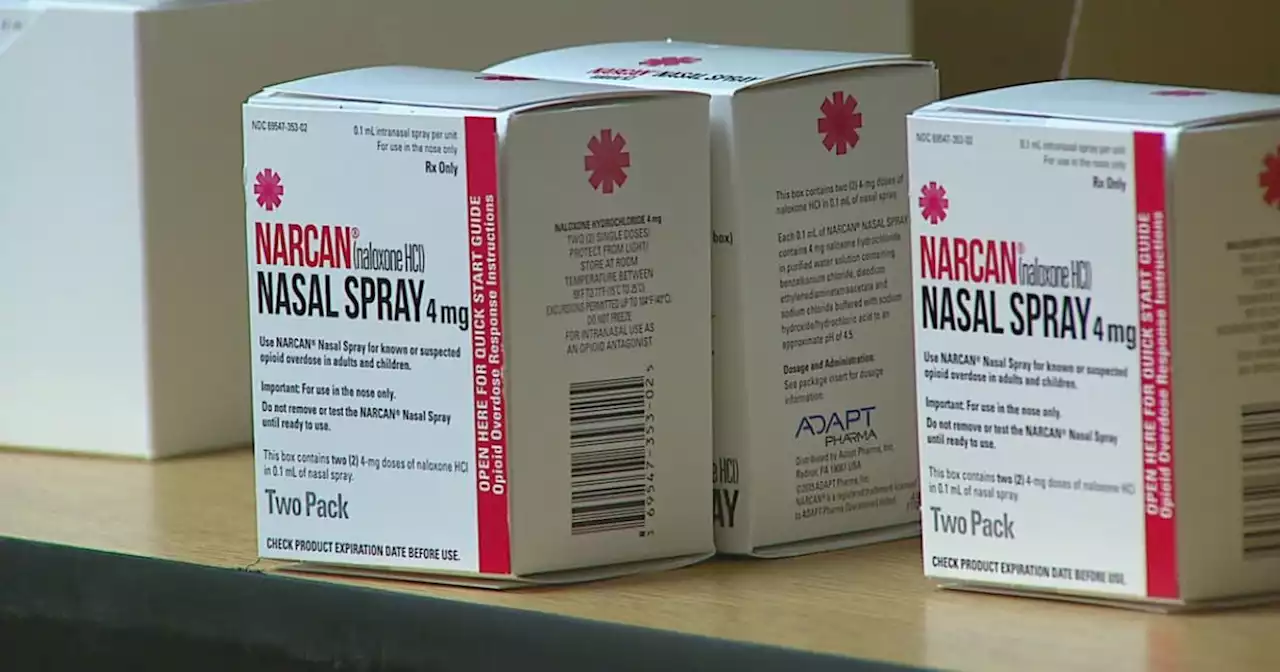 Narcan maker says anti-opioid nasal spray will soon be available over the counterFDA to give priority review to nonprescription form of naloxone, a life-saving drug used to treat suspected drug overdoses.
Narcan maker says anti-opioid nasal spray will soon be available over the counterFDA to give priority review to nonprescription form of naloxone, a life-saving drug used to treat suspected drug overdoses.
続きを読む »
 FDA to review Emergent's request to approve over-the-counter NarcanShares of Emergent BioSolutions Inc. undefined were up 1.7% in premarket trading on Tuesday after the company said the Food and Drug Administration is...
FDA to review Emergent's request to approve over-the-counter NarcanShares of Emergent BioSolutions Inc. undefined were up 1.7% in premarket trading on Tuesday after the company said the Food and Drug Administration is...
続きを読む »
 FDA grants priority review for over-the-counter naloxone nasal spray to treat opioid overdoseContract drugmaker Emergent Biosolutions said on Tuesday its over-the-counter naloxone-based nasal spray as a treatment for suspected opioid overdose would be reviewed on a priority basis by the FDA.
FDA grants priority review for over-the-counter naloxone nasal spray to treat opioid overdoseContract drugmaker Emergent Biosolutions said on Tuesday its over-the-counter naloxone-based nasal spray as a treatment for suspected opioid overdose would be reviewed on a priority basis by the FDA.
続きを読む »
 U.S. FDA to review Emergent's OTC opioid overdose drug on priorityContract drugmaker Emergent Biosolutions said on Tuesday its over-the-counter nasal spray as a treatment for suspected opioid overdose would be reviewed on a priority basis by the U.S. health regulator.
U.S. FDA to review Emergent's OTC opioid overdose drug on priorityContract drugmaker Emergent Biosolutions said on Tuesday its over-the-counter nasal spray as a treatment for suspected opioid overdose would be reviewed on a priority basis by the U.S. health regulator.
続きを読む »
 WSJ News Exclusive | Narcan Maker Gets Fast-Tracked for Over-the-Counter Nasal SprayThe market for over-the-counter opioid-reversal drugs is heating up, with Narcan maker’s application for approval getting priority review from the FDA
WSJ News Exclusive | Narcan Maker Gets Fast-Tracked for Over-the-Counter Nasal SprayThe market for over-the-counter opioid-reversal drugs is heating up, with Narcan maker’s application for approval getting priority review from the FDA
続きを読む »
