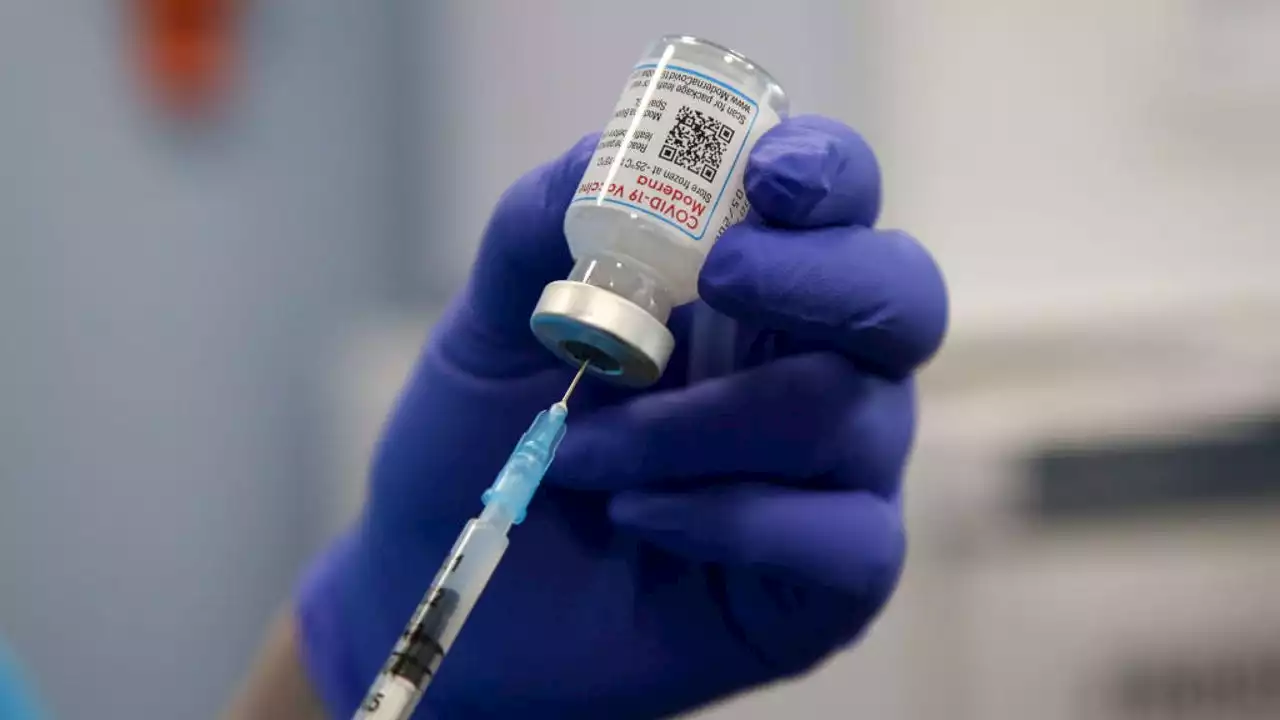
Moderna announced Thursday that the company has asked the Food and Drug Administration to authorize a low-dose version of its COVID-19 vaccine as the first vaccine for children younger than age 5.
Moderna says its vaccine appears to be about 51 percent effective for children ages 6 months to less than 2 years, and 37 percent effective for those ages 2 to less than 6 years.
The vaccine appears to be about 51 percent effective for children ages 6 months to less than 2 years, and 37 percent effective for those ages 2 to less than 6 years, the company says. But “the levels of antibodies that we see clearly shows that we should have very good protection against severe disease and hospitalization, which obviously is what counts most,” Burton said.
日本 最新ニュース, 日本 見出し
Similar News:他のニュース ソースから収集した、これに似たニュース記事を読むこともできます。
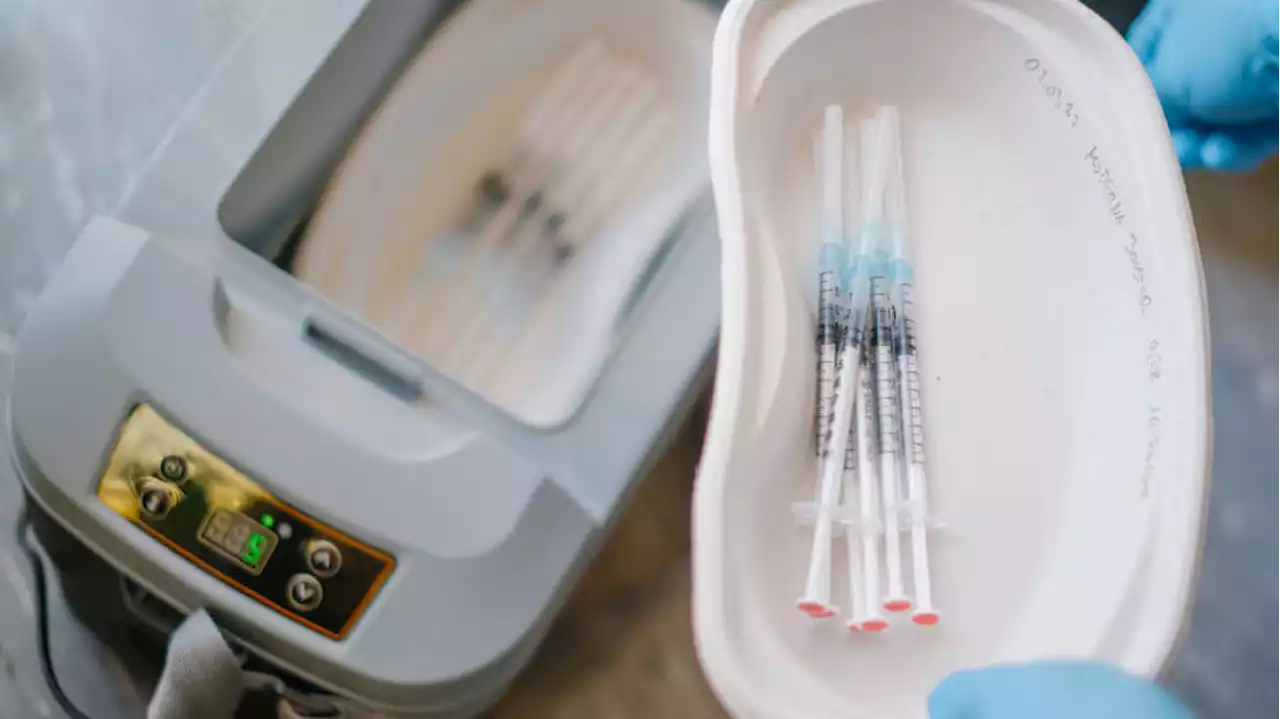
 Moderna asks FDA to approve COVID-19 vaccine for kids under 6Moderna is asking U.S. regulators to approve its low-dose COVID-19 vaccine for children younger than 6, potentially opening shots for millions of tots by summer.
Moderna asks FDA to approve COVID-19 vaccine for kids under 6Moderna is asking U.S. regulators to approve its low-dose COVID-19 vaccine for children younger than 6, potentially opening shots for millions of tots by summer.
続きを読む »
 Moderna Asks FDA to Approve Its COVID-19 Shot for Kids Under 6Moderna on Thursday asked U.S. regulators to authorize low doses of its COVID-19 vaccine for children younger than 6, a long-awaited move toward potentially opening shots for millions of tots by summer.
Moderna Asks FDA to Approve Its COVID-19 Shot for Kids Under 6Moderna on Thursday asked U.S. regulators to authorize low doses of its COVID-19 vaccine for children younger than 6, a long-awaited move toward potentially opening shots for millions of tots by summer.
続きを読む »
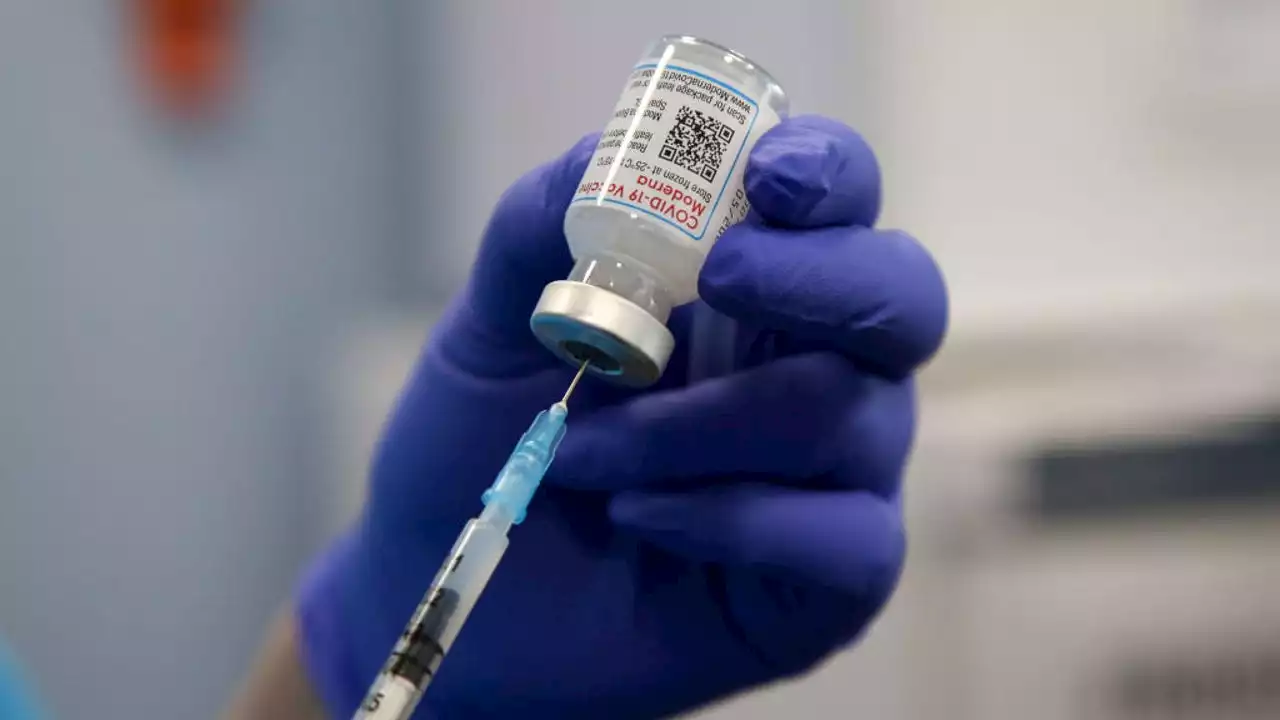
 Moderna asks FDA to authorize first COVID-19 vaccine for very young childrenThe company says a low-dose version of its vaccine triggers an immune response in children ages 6 months to less than 6 years equivalent to what has protected older children and adults.
Moderna asks FDA to authorize first COVID-19 vaccine for very young childrenThe company says a low-dose version of its vaccine triggers an immune response in children ages 6 months to less than 6 years equivalent to what has protected older children and adults.
続きを読む »
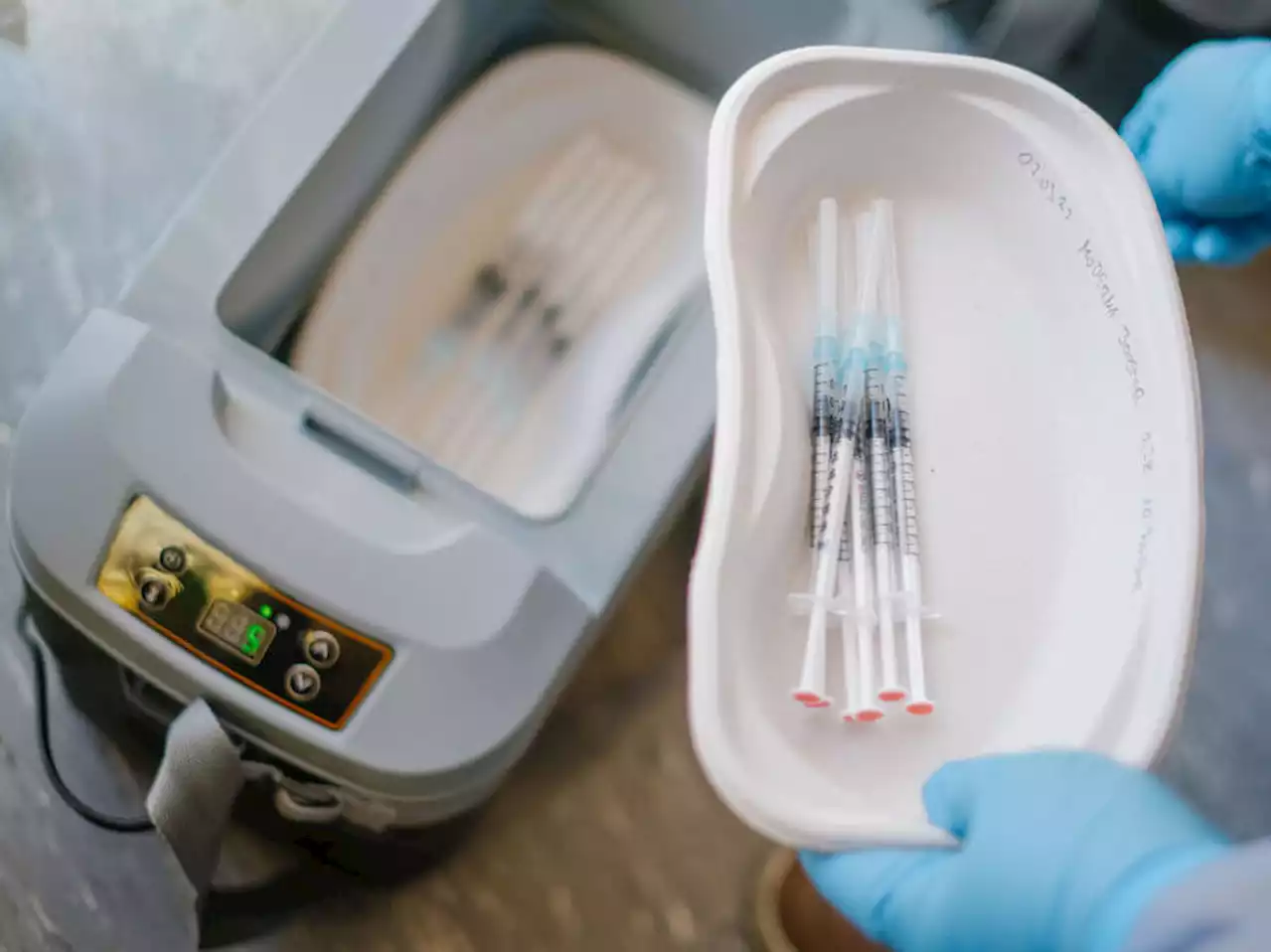
 Moderna asks FDA to approve COVID-19 vaccine for kids under 6Moderna is asking U.S. regulators to approve its low-dose COVID-19 vaccine for children younger than 6, potentially opening shots for millions of tots by summer.
Moderna asks FDA to approve COVID-19 vaccine for kids under 6Moderna is asking U.S. regulators to approve its low-dose COVID-19 vaccine for children younger than 6, potentially opening shots for millions of tots by summer.
続きを読む »
 Moderna asks FDA for authorization of its COVID-19 vaccine for children under 6The submission marks a hopeful development in the long journey for parents who are desperate to get their young kids vaccinated.
Moderna asks FDA for authorization of its COVID-19 vaccine for children under 6The submission marks a hopeful development in the long journey for parents who are desperate to get their young kids vaccinated.
続きを読む »
 Moderna Asks FDA to Clear Its Covid-19 Vaccine for Young ChildrenModerna asked the FDA to authorize its Covid-19 vaccine for children 6 months to 5 years. If regulators agree, vaccinations could begin by summer.
Moderna Asks FDA to Clear Its Covid-19 Vaccine for Young ChildrenModerna asked the FDA to authorize its Covid-19 vaccine for children 6 months to 5 years. If regulators agree, vaccinations could begin by summer.
続きを読む »