The FDA on Friday approved the first pill to treat postpartum depression, a condition that data shows affects around 1 in 7 women in the United States.
The Good Day team discusses the condition and the warning signs for mothers after delivery.The Food and Drug Administration on Friday approved the first pill to treat postpartum depression, a condition that data shows affects around 1 in 7 women in the United States., could be a gamechanger for treating postpartum depression and other depressive disorders after clinical trials found the 14-day daily pill began alleviating symptoms in a matter of days.
“It’s really great that this medication is coming out because if it is able to treat postpartum depression that effectively, then it would alleviate a lot of suffering,” said Dr. Nirmaljit Dhami, medical director of the Inpatient Perinatal Psychiatry Unit at El Camino Health’s Scrivner Center for Mental Health & Addiction Services in Mountain View, California.
Neuroactive steroids are molecules that naturally occur in the body and are important for managing stress in the brain, said the study’s principal investigator Dr. Kristina Deligiannidis, a professor at the Institute of Behavioral Science at the Feinstein Institutes for Medical Research in New York. The most common side effects reported included drowsiness, dizziness and sedation. But no participants experienced withdrawal symptoms,How is this drug different from other antidepressants?
日本 最新ニュース, 日本 見出し
Similar News:他のニュース ソースから収集した、これに似たニュース記事を読むこともできます。
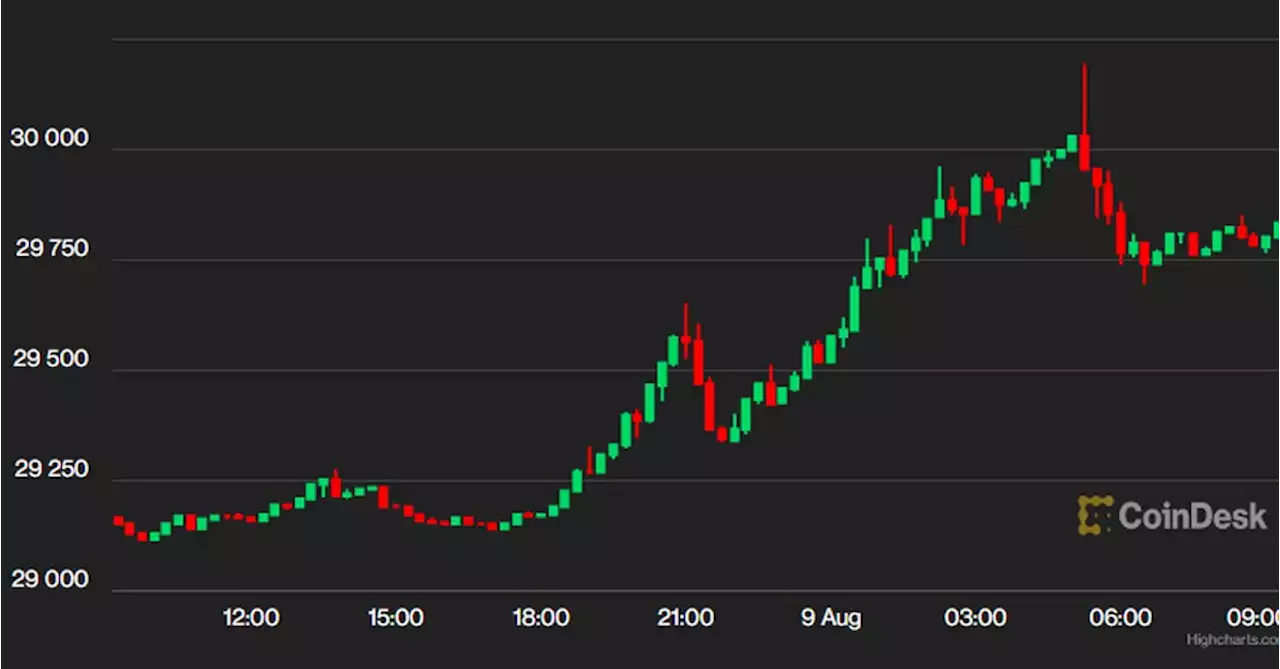 First Mover Asia: First $30K, Then $40K, but Bitcoin Needs Volatility FirstBitcoin's volatility remains low, but it is outperforming most digital asset funds this year. Traders are looking for catalysts that could lead to a rebound, such as ETF narratives or industry-transforming events.
First Mover Asia: First $30K, Then $40K, but Bitcoin Needs Volatility FirstBitcoin's volatility remains low, but it is outperforming most digital asset funds this year. Traders are looking for catalysts that could lead to a rebound, such as ETF narratives or industry-transforming events.
続きを読む »
FDA Approves First Pill to Treat Postpartum DepressionThe FDA has approved a breakthrough pill for the treatment of postpartum depression. The pill, which takes effect in as little as three days, could be available for use by November. A recent study showed significant improvements in depressive symptoms for women taking a 50-milligram dose for two weeks.
続きを読む »
 FDA Approves First Pill for Postpartum DepressionThe FDA has granted approval for the first pill to treat postpartum depression. The pill, called Zurzuvae, has shown significant improvement in depressive symptoms compared to a placebo group in a recent study. This is a major breakthrough as the only other approved therapy for postpartum depression is an expensive IV drug that requires long infusions in hospital settings.
FDA Approves First Pill for Postpartum DepressionThe FDA has granted approval for the first pill to treat postpartum depression. The pill, called Zurzuvae, has shown significant improvement in depressive symptoms compared to a placebo group in a recent study. This is a major breakthrough as the only other approved therapy for postpartum depression is an expensive IV drug that requires long infusions in hospital settings.
続きを読む »
 The FDA Officially Approved The First Pill For Postpartum Depression — And It's 'Highly Effective'Zuranolone, a new drug used to treat postpartum depression in new moms, could be approved by the FDA as soon as August. Clinical trials conducted in 2021 showed that the drug was effective and safe. Sahar McMahon, a member of the trial study, said to CNN of her experience taking zuranolone, 'It saved my life. It saved my marriage. It saved my kids.'
The FDA Officially Approved The First Pill For Postpartum Depression — And It's 'Highly Effective'Zuranolone, a new drug used to treat postpartum depression in new moms, could be approved by the FDA as soon as August. Clinical trials conducted in 2021 showed that the drug was effective and safe. Sahar McMahon, a member of the trial study, said to CNN of her experience taking zuranolone, 'It saved my life. It saved my marriage. It saved my kids.'
続きを読む »
 FDA approves first oral medication to treat postpartum depressionThe FDA has approved the oral medication Zurzuvae as the first treatment option for postpartum depression. Previously, the only available treatment was through an I.V. injection. The medication is taken over a 14-day period and offers hope for mothers suffering from depression, anxiety, and intrusive thoughts after childbirth.
FDA approves first oral medication to treat postpartum depressionThe FDA has approved the oral medication Zurzuvae as the first treatment option for postpartum depression. Previously, the only available treatment was through an I.V. injection. The medication is taken over a 14-day period and offers hope for mothers suffering from depression, anxiety, and intrusive thoughts after childbirth.
続きを読む »
