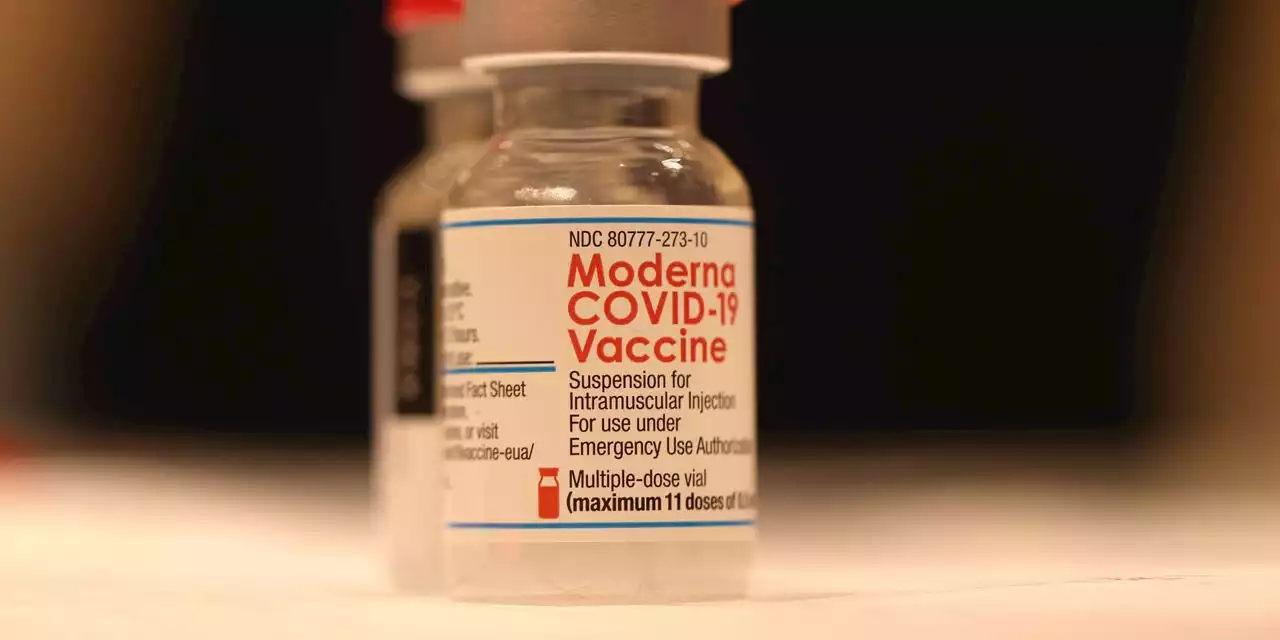
The FDA's outside experts voted unanimously that Moderna’s COVID vaccine is safe and effective enough to give kids ages 6 to 17.
The Food and Drug Administration’s outside experts voted unanimously that Moderna’s MRNA, +3.78% vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer’s vaccine.
The Massachusetts company is seeking clearance for two doses, and plans to later offer a booster. Tuesday’s vote was only for two doses — full-strength for 12-17 and half-sized doses for those 6-11. In their review, FDA scientists said there were no confirmed cases of the heart inflammation in Moderna’s kid studies. But experts say the studies may have had too few participants for a rare side effect like that to appear.
The FDA analysis concluded that two doses of Moderna are effective in preventing symptomatic COVID-19 illness in teens and younger kids, with the levels of virus-fighting antibodies comparable to those developed in young adults.
日本 最新ニュース, 日本 見出し
Similar News:他のニュース ソースから収集した、これに似たニュース記事を読むこともできます。
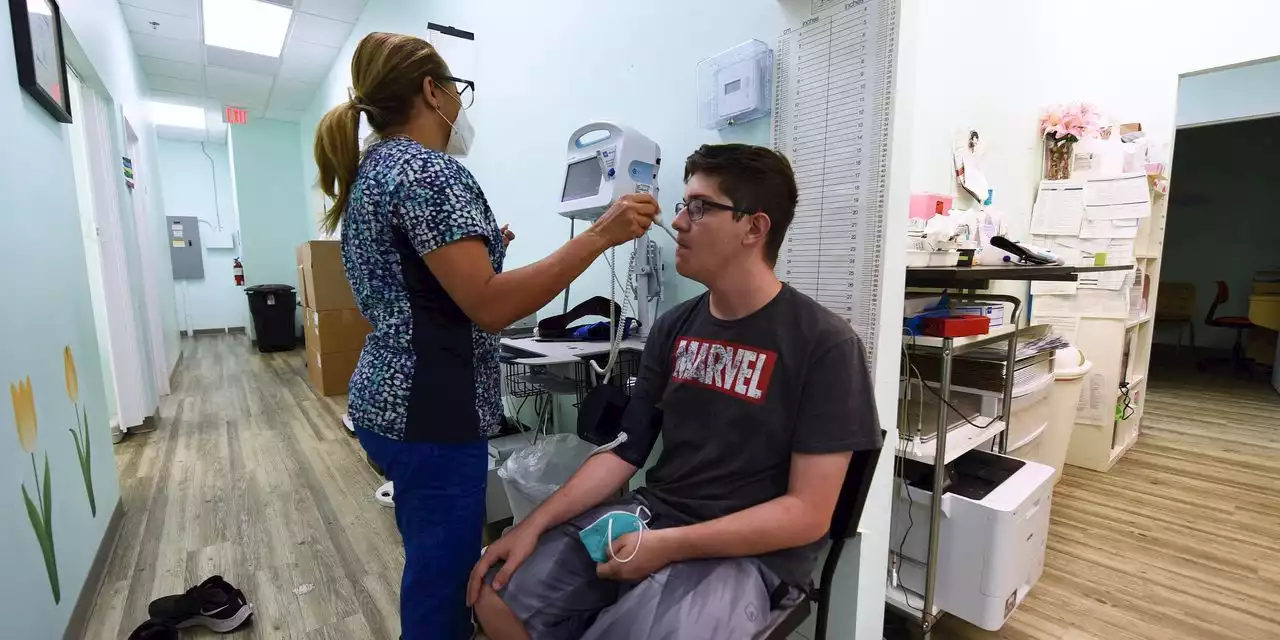 FDA Advisers Considering Moderna’s Covid-19 Vaccine for Ages 6 to 17An advisory committee is set to vote Tuesday on whether to recommend that the Food and Drug Administration authorize use of the Moderna vaccine for 6- to 17-year-olds.
FDA Advisers Considering Moderna’s Covid-19 Vaccine for Ages 6 to 17An advisory committee is set to vote Tuesday on whether to recommend that the Food and Drug Administration authorize use of the Moderna vaccine for 6- to 17-year-olds.
続きを読む »
 Dogs Can Sniff Out COVID-19 and Signs of Long COVID, Studies SuggestAlmost any dog can be trained to do this type of detection in a matter of weeks, researchers say
Dogs Can Sniff Out COVID-19 and Signs of Long COVID, Studies SuggestAlmost any dog can be trained to do this type of detection in a matter of weeks, researchers say
続きを読む »
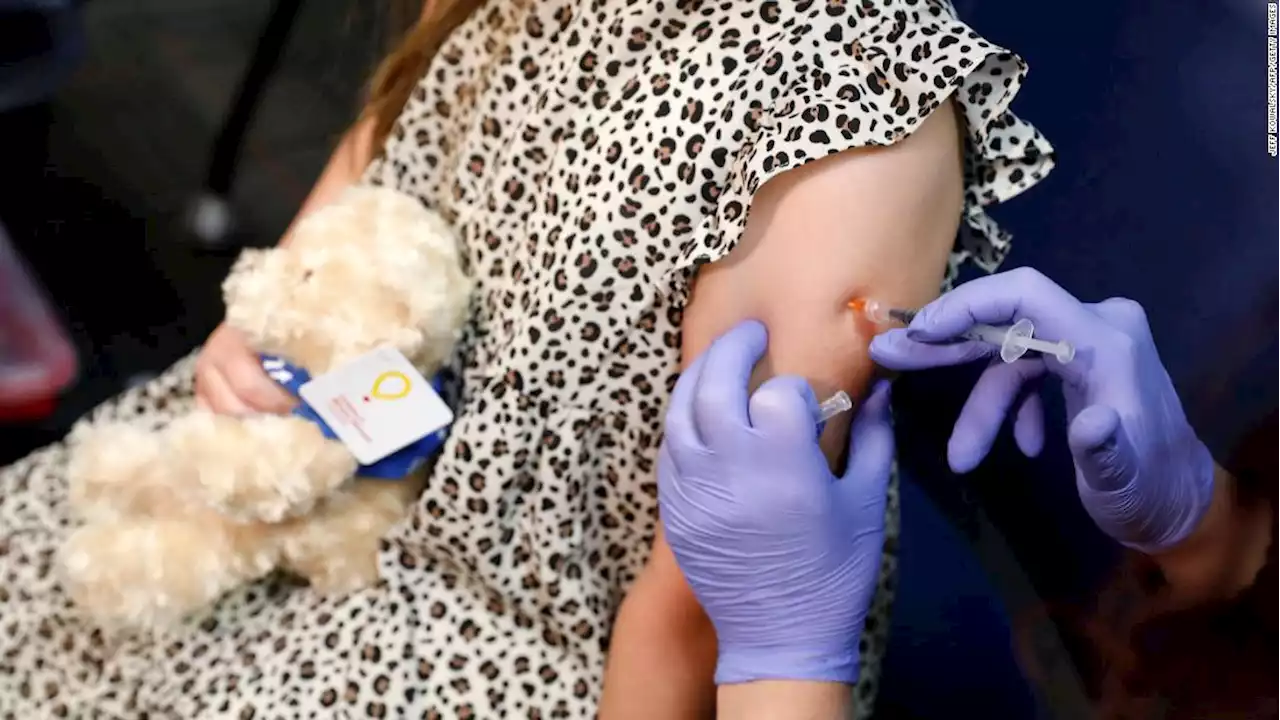 FDA advisers to weigh expanding Covid-19 vaccines to younger childrenSeveral months after older children became eligible to get vaccinated against Covid-19, the United States might be just days away from offering vaccines to those younger than 5.
FDA advisers to weigh expanding Covid-19 vaccines to younger childrenSeveral months after older children became eligible to get vaccinated against Covid-19, the United States might be just days away from offering vaccines to those younger than 5.
続きを読む »
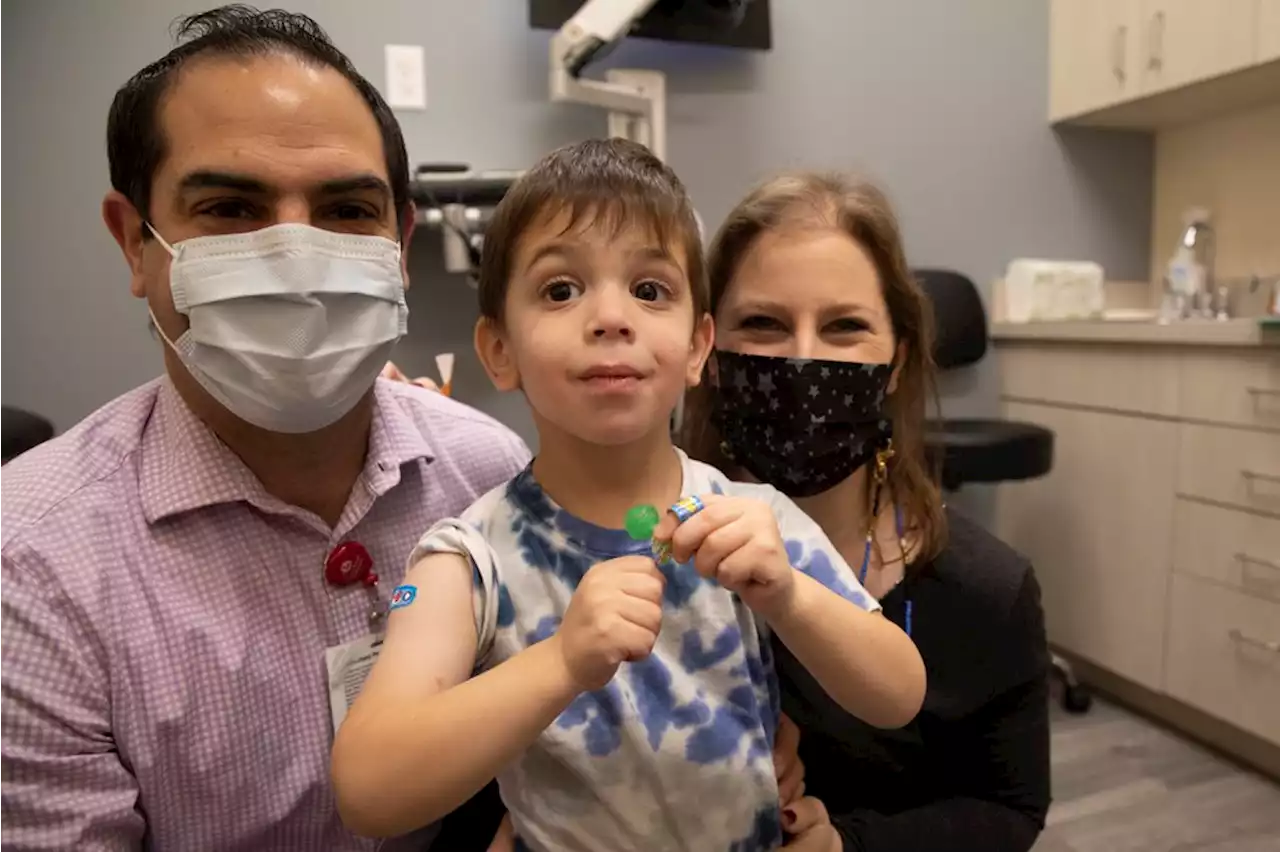 FDA advisers back Moderna’s COVID-19 vaccine for teens, younger kidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens.
FDA advisers back Moderna’s COVID-19 vaccine for teens, younger kidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens.
続きを読む »
 FDA advisers back Moderna's COVID-19 vaccine for older kidsA government advisory panel has endorsed a second brand of COVID-19 vaccine for school-age children and teens. The expert panel agreed Tuesday that the vaccine made by Moderna is safe and effective enough to give to U.S. kids ages 6 to 17.
FDA advisers back Moderna's COVID-19 vaccine for older kidsA government advisory panel has endorsed a second brand of COVID-19 vaccine for school-age children and teens. The expert panel agreed Tuesday that the vaccine made by Moderna is safe and effective enough to give to U.S. kids ages 6 to 17.
続きを読む »
 FDA Advisers Back Moderna's COVID-19 Vaccine for Older KidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens. The Food and Drug Administration’s outside experts voted unanimously that Moderna’s vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer’s vaccine. The same FDA…
FDA Advisers Back Moderna's COVID-19 Vaccine for Older KidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens. The Food and Drug Administration’s outside experts voted unanimously that Moderna’s vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer’s vaccine. The same FDA…
続きを読む »