The U.S. government on Thursday will lay out its long-awaited plan to ban menthol cigarettes and flavored cigars, which have taken a disproportionate toll on Black smokers and other minorities.
FILE - Menthol cigarettes and other tobacco products are displayed at a store in San Francisco on May 17, 2018. The U.S. government is set to release its long-awaited plan to ban menthol cigarettes and flavored cigars. On Thursday, April 28, 2022, Food and Drug Administration Commissioner Robert Califf previewed the announcement in congressional testimony, saying the proposal would reduce disease and death among smokers and help many quit. – The U.S.
The Food and Drug Administration said eliminating menthol cigarettes could prevent between 300,000 and 650,000 smoking deaths over 40 years. The FDA will also seek to ban menthol and dozens of other flavors like grape and strawberry from cigars, which are increasingly popular with young people, especially Black teens.
“This is the first time there’s been support from an administration,” said Mitch Zeller, who recently retired after nine years leading FDA’s tobacco center. “If these rules are finalized they become the law of the land and it becomes illegal for menthol cigarettes and flavored cigars to be sold.”— for and against the ban — met with Biden administration officials to try and influence the proposed rule, which would wipe out billions in tobacco sales.
日本 最新ニュース, 日本 見出し
Similar News:他のニュース ソースから収集した、これに似たニュース記事を読むこともできます。
 FDA Approves First COVID Treatment for Use in KidsThe FDA has approved the antiviral remdesivir as the first COVID-19 treatment for young children.
FDA Approves First COVID Treatment for Use in KidsThe FDA has approved the antiviral remdesivir as the first COVID-19 treatment for young children.
続きを読む »
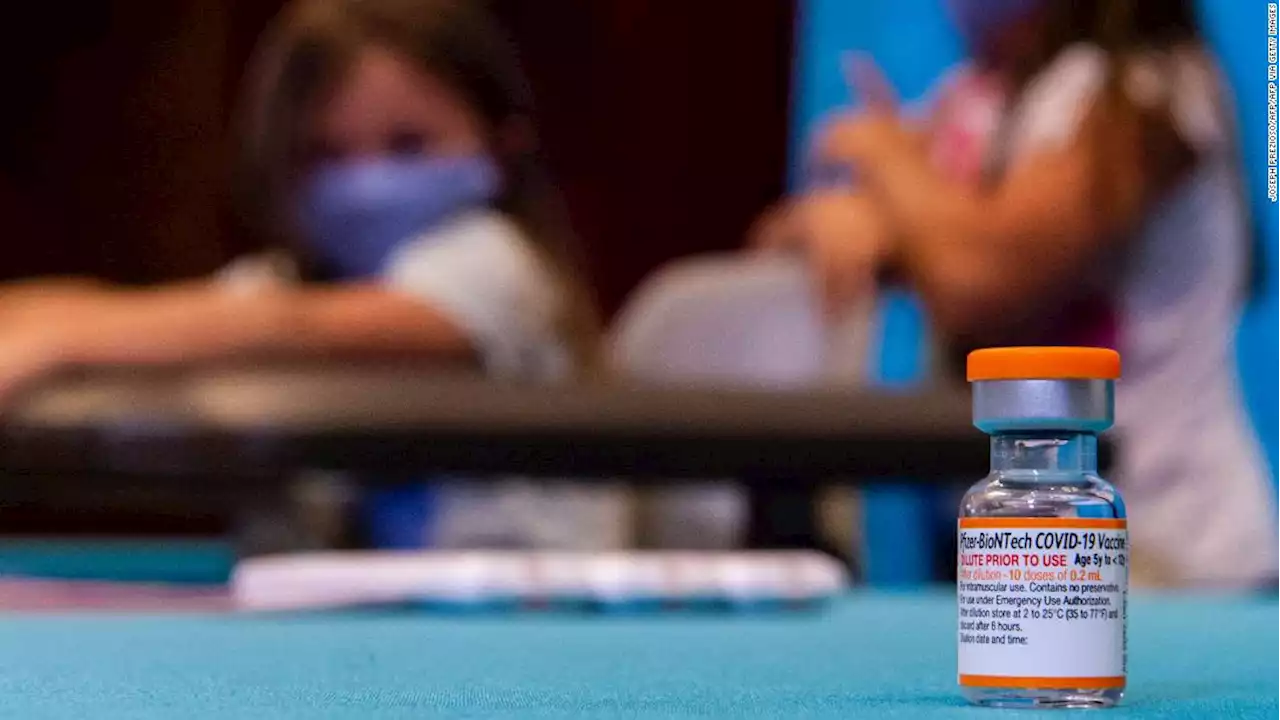 Pfizer requests FDA authorization for Covid-19 booster for kids 5 through 11Pfizer and BioNTech said Tuesday that they have submitted an application to the US Food and Drug Administration for emergency use authorization of a booster dose of Covid-19 vaccine for children 5 through 11.
Pfizer requests FDA authorization for Covid-19 booster for kids 5 through 11Pfizer and BioNTech said Tuesday that they have submitted an application to the US Food and Drug Administration for emergency use authorization of a booster dose of Covid-19 vaccine for children 5 through 11.
続きを読む »
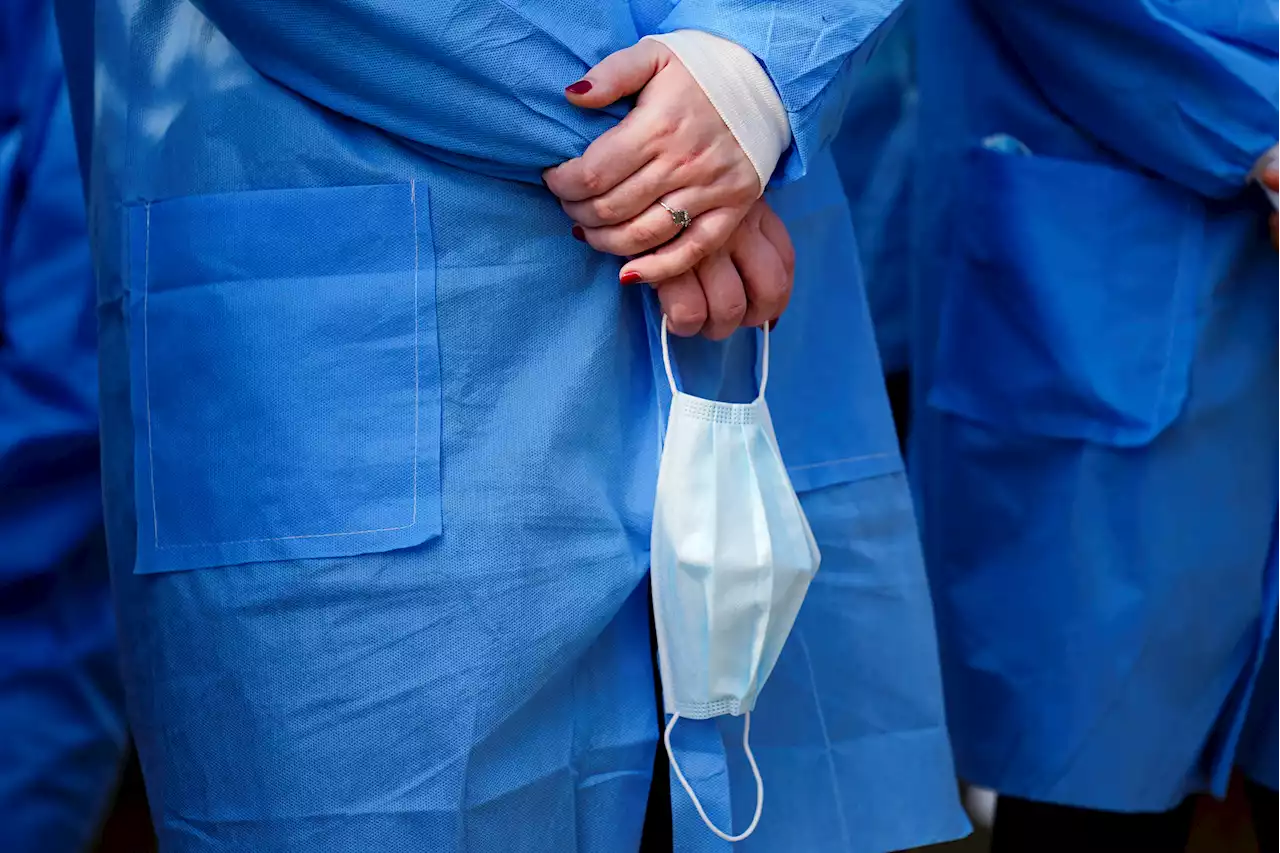 Pfizer Asks FDA to Authorize Third Dose for Children 5 to 11 Years OldPfizer on Tuesday asked the Food and Drug Administration to authorize a third dose of its COVID vaccine for children ages 5- to 11-years-old. The application comes after Pfizer released data earlier this month from a small lab study of blood samples from 30 kids in the age group, which showed a 36-fold increase in antibody levels against the omicron variant…
Pfizer Asks FDA to Authorize Third Dose for Children 5 to 11 Years OldPfizer on Tuesday asked the Food and Drug Administration to authorize a third dose of its COVID vaccine for children ages 5- to 11-years-old. The application comes after Pfizer released data earlier this month from a small lab study of blood samples from 30 kids in the age group, which showed a 36-fold increase in antibody levels against the omicron variant…
続きを読む »
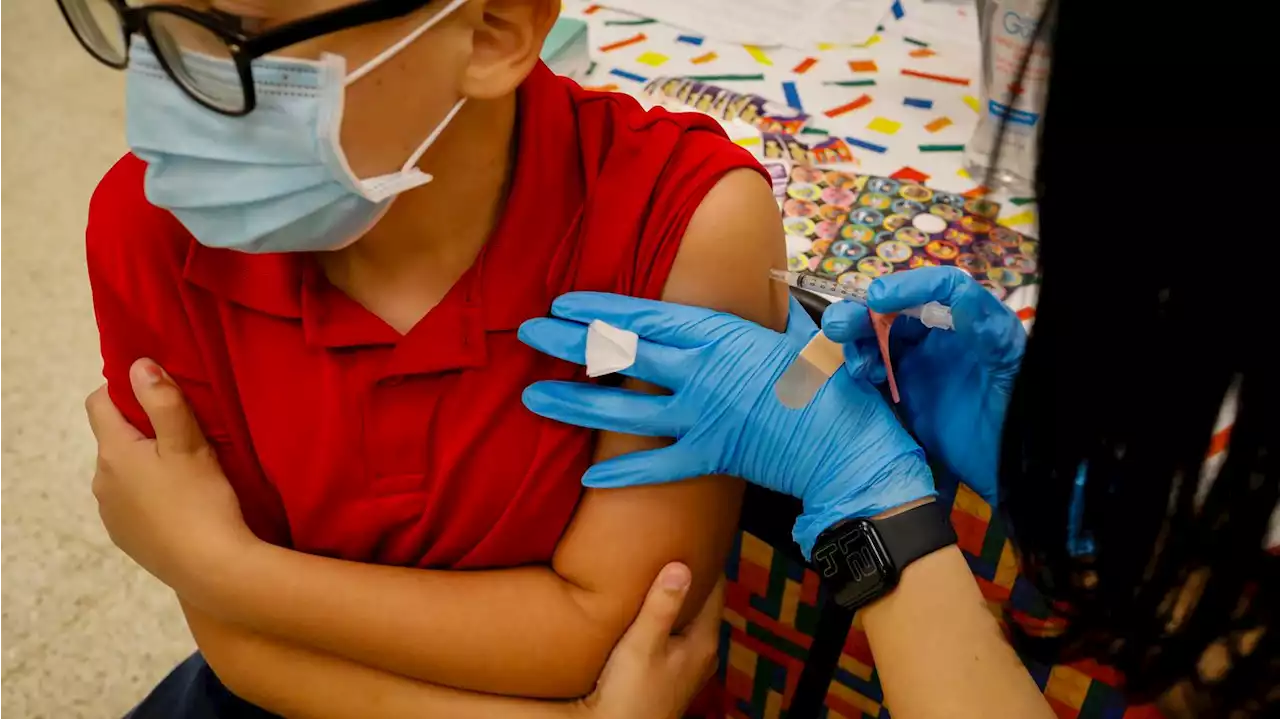 Pfizer asks FDA to authorize COVID booster for children 5 to 11If approved, it would be the first booster accessible to children of this age group, who were hit hard by Omicron.
Pfizer asks FDA to authorize COVID booster for children 5 to 11If approved, it would be the first booster accessible to children of this age group, who were hit hard by Omicron.
続きを読む »
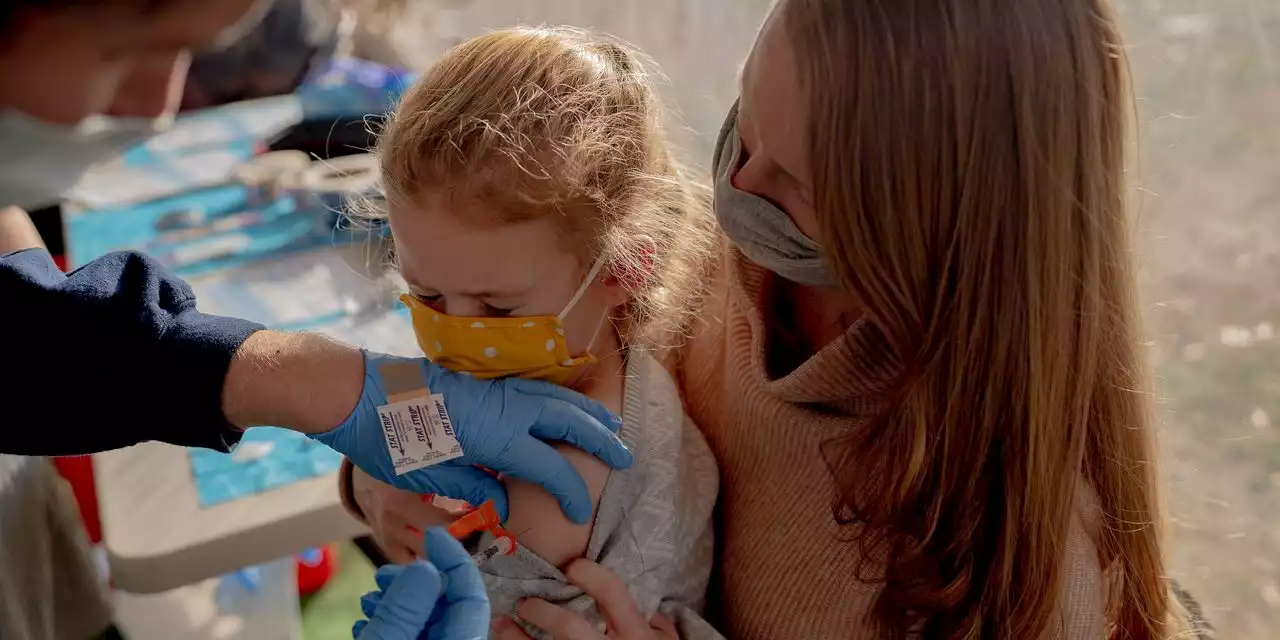 Pfizer Asks FDA to Authorize Covid-19 Booster for 5- to 11-Year-OldsPfizer Inc. and partner BioNTech SE said earlier this month that a third shot safely generated a strong immune response in the youngsters.
Pfizer Asks FDA to Authorize Covid-19 Booster for 5- to 11-Year-OldsPfizer Inc. and partner BioNTech SE said earlier this month that a third shot safely generated a strong immune response in the youngsters.
続きを読む »
 Pfizer-BioNTech seeks FDA authorization for a COVID booster shot for kids 5-11Pfizer and BioNTech have requested that the Food and Drug Administration authorize a COVID-19 booster shot for children ages 5-11.
Pfizer-BioNTech seeks FDA authorization for a COVID booster shot for kids 5-11Pfizer and BioNTech have requested that the Food and Drug Administration authorize a COVID-19 booster shot for children ages 5-11.
続きを読む »
