WASHINGTON (AP) — COVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they don’t work against the omicron variant that...
U.S. health officials say COVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they don’t work against the omicron variant
The regulatory move was expected because both drugmakers had said the infusion drugs are less able to target omicron due to its mutations. Still, the federal action could trigger pushback from some Republican governors who have continued promoting the drugs against the advice of health experts.the treatment playbook for COVID-19 in recent weeks.
The U.S. government temporarily stopped distributing the two drugs in late December, as omicron was racing across the country to become the dominant variant. But officials resumed distribution after complaints from Republican governors, including Florida’s Ron DeSantis, who claimed that the drugs continued to help some omicron patients.
Since early January, the U.S. government has shipped enough doses of the two antibodies to treat more than 300,000 patients. On Friday, the FDA expanded the antiviral’s approval to include adults and children with early COVID-19 who face a high risk of ending up in the hospital. Remdesivir previously had been limited to hospitalized patients.
日本 最新ニュース, 日本 見出し
Similar News:他のニュース ソースから収集した、これに似たニュース記事を読むこともできます。
 FDA Curbs Use of COVID-19 Antibody Drugs Sidelined by OmicronU.S. health officials say COVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they are unlikely to work against the omicron variant.
FDA Curbs Use of COVID-19 Antibody Drugs Sidelined by OmicronU.S. health officials say COVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they are unlikely to work against the omicron variant.
続きを読む »
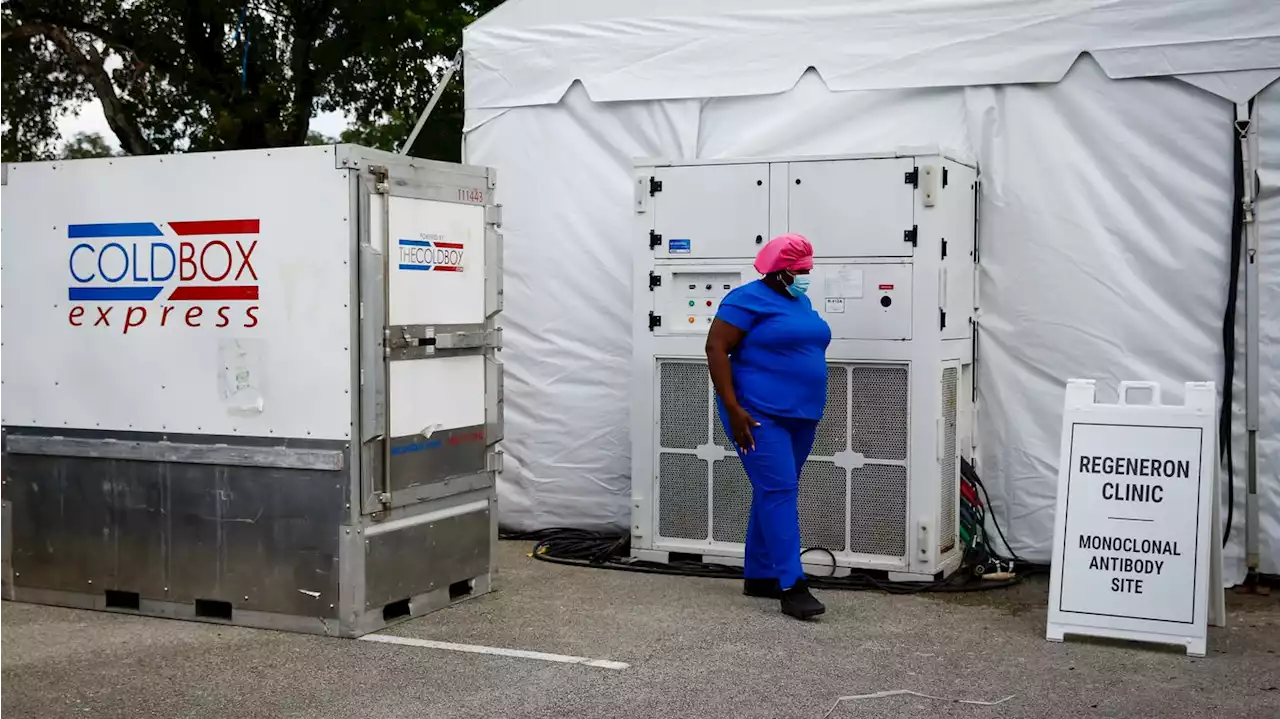 FDA limits use of Regeneron and Lilly COVID antibody treatmentsThe FDA said 'data show these treatments are highly unlikely to be active' against Omicron.
FDA limits use of Regeneron and Lilly COVID antibody treatmentsThe FDA said 'data show these treatments are highly unlikely to be active' against Omicron.
続きを読む »
 Pfizer and BioNTech launch study of omicron vaccine, and FDA halts use of two COVID antibody treatmentsOne day after the U.S. Food and Drug Administration said it's halting the use of antibody drugs as COVID-19 treatments because they don't work on the highly...
Pfizer and BioNTech launch study of omicron vaccine, and FDA halts use of two COVID antibody treatmentsOne day after the U.S. Food and Drug Administration said it's halting the use of antibody drugs as COVID-19 treatments because they don't work on the highly...
続きを読む »
 FDA halts use of monoclonal antibody drugs from Regeneron, Eli Lilly that don't work vs. omicronCOVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they don't work against the omicron variant, U.S. health officials said.
FDA halts use of monoclonal antibody drugs from Regeneron, Eli Lilly that don't work vs. omicronCOVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they don't work against the omicron variant, U.S. health officials said.
続きを読む »
 FDA halts use of antibody drugs that don’t work against omicronCOVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they don’t work against the omicron variant that now accounts for nearly all U.S. infections, U.S. health regulators said Monday.
FDA halts use of antibody drugs that don’t work against omicronCOVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they don’t work against the omicron variant that now accounts for nearly all U.S. infections, U.S. health regulators said Monday.
続きを読む »
 FDA halts use of monoclonal antibody drugs from Regeneron, Eli Lilly that don't work vs. omicronCOVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they don't work against the omicron variant, U.S. health officials said.
FDA halts use of monoclonal antibody drugs from Regeneron, Eli Lilly that don't work vs. omicronCOVID-19 antibody drugs from Regeneron and Eli Lilly should no longer be used because they don't work against the omicron variant, U.S. health officials said.
続きを読む »
