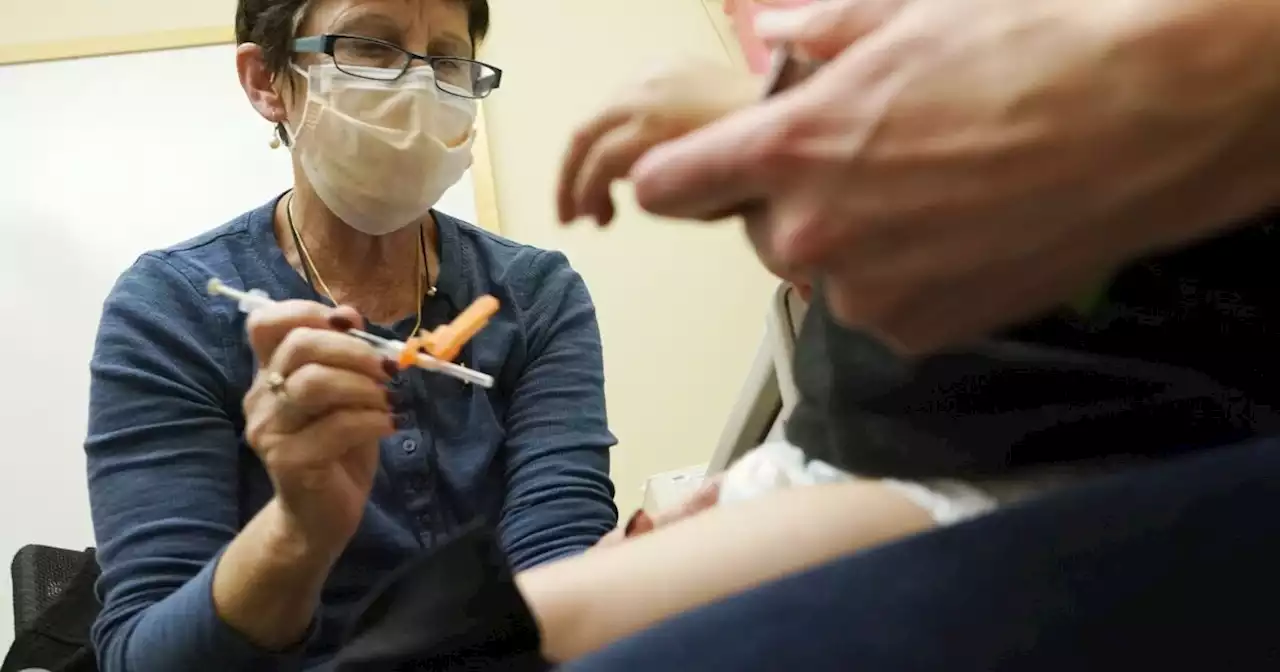
U.S. regulators have cleared doses of the updated COVID-19 vaccines to children younger than 5
U.S. regulators on Thursday cleared doses of the updated COVID-19 vaccines for children younger than age 5.
--Pfizer’s vaccine requires three initial doses for tots under age 5 -- and those who haven’t finished that vaccination series will get the original formula for the first two shots and the omicron-targeted version for their third shot. The Centers for Disease Control and Prevention is expected to sign off soon, the final step for shots to begin.
日本 最新ニュース, 日本 見出し
Similar News:他のニュース ソースから収集した、これに似たニュース記事を読むこともできます。
 FDA authorizes updated COVID shots for kids as young as 6 months oldThe FDA is encouraging families to get young children vaccinated ahead of the holidays.
FDA authorizes updated COVID shots for kids as young as 6 months oldThe FDA is encouraging families to get young children vaccinated ahead of the holidays.
続きを読む »
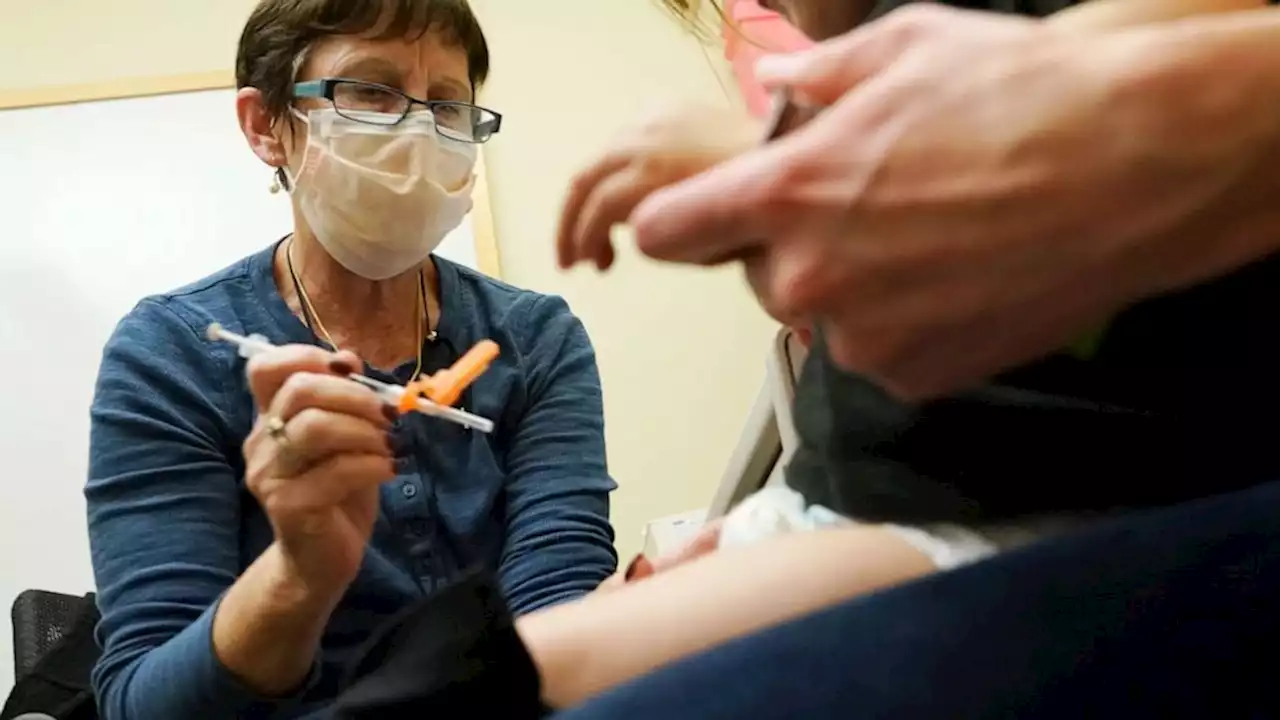 FDA authorizes updated COVID-19 boosters for kids under 5The U.S. Food and Drug Administration authorized bivalent COVID-19 boosters for children between ages 6 months and 4 years Thursday.
FDA authorizes updated COVID-19 boosters for kids under 5The U.S. Food and Drug Administration authorized bivalent COVID-19 boosters for children between ages 6 months and 4 years Thursday.
続きを読む »
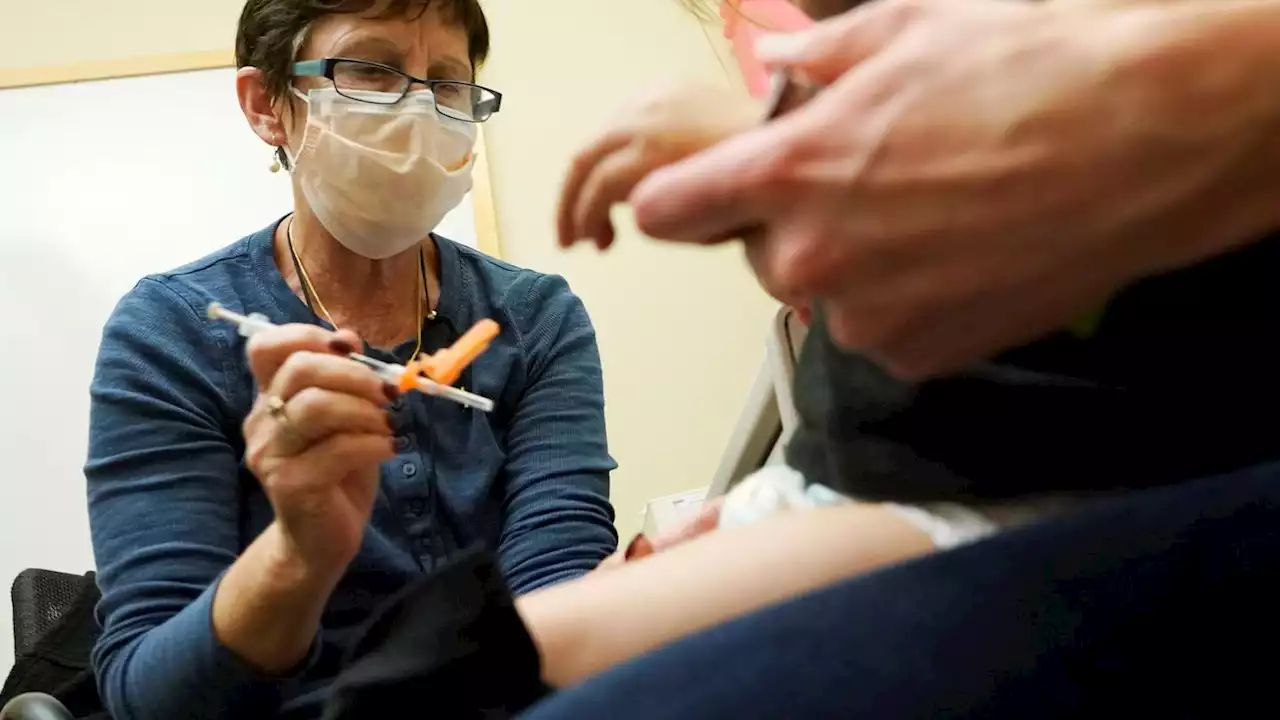 FDA clears updated COVID-19 vaccines for kids under age 5U.S. regulators have cleared doses of the updated COVID-19 vaccines to children younger than 5
FDA clears updated COVID-19 vaccines for kids under age 5U.S. regulators have cleared doses of the updated COVID-19 vaccines to children younger than 5
続きを読む »
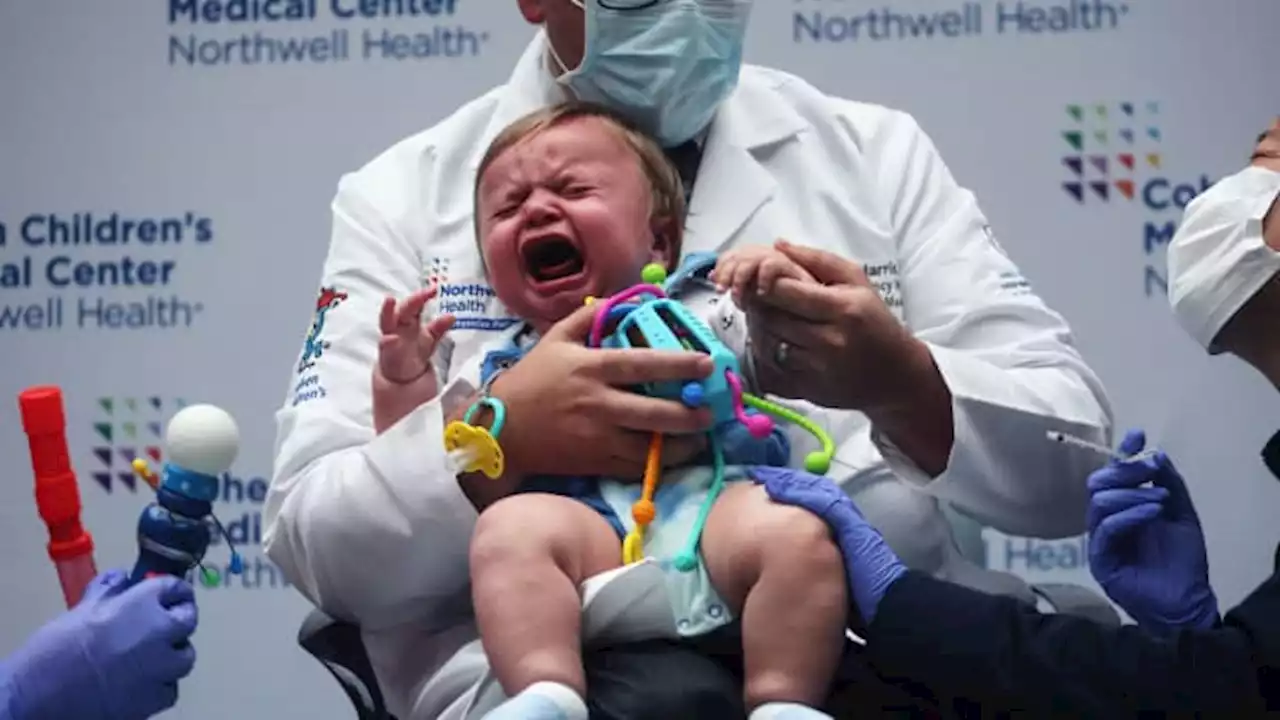 FDA authorizes Covid omicron vaccines for children as young as 6 months old
FDA authorizes Covid omicron vaccines for children as young as 6 months old
続きを読む »
 Pfizer partners with Clear Creek Bio to develop oral COVID-19 drugPfizer Inc and Clear Creek Bio Inc on Tuesday announced a collaboration to identify a potential drug candidate and develop a new class of oral treatment against COVID-19, as Pfizer seeks to expand its anti-infective pipeline.
Pfizer partners with Clear Creek Bio to develop oral COVID-19 drugPfizer Inc and Clear Creek Bio Inc on Tuesday announced a collaboration to identify a potential drug candidate and develop a new class of oral treatment against COVID-19, as Pfizer seeks to expand its anti-infective pipeline.
続きを読む »