According to the FDA notice, the Sacramento-based Blue Diamond Growers initiated the recall after an internal sampling was positive for salmonella on the implicated lots.
The Food and Drug Administration announced that Blue Diamond voluntarily recalled close to 350,000 pounds of almonds due to potential salmonella contamination.
The FDA said that the 347,650 pounds of the affected product are labeled whole brown almonds and were sold in bulk.The FDA notice said that the recalled products were distributed in California, Colorado, and Illinois and sold internationally in Germany, Morocco, and Canada.
日本 最新ニュース, 日本 見出し
Similar News:他のニュース ソースから収集した、これに似たニュース記事を読むこともできます。
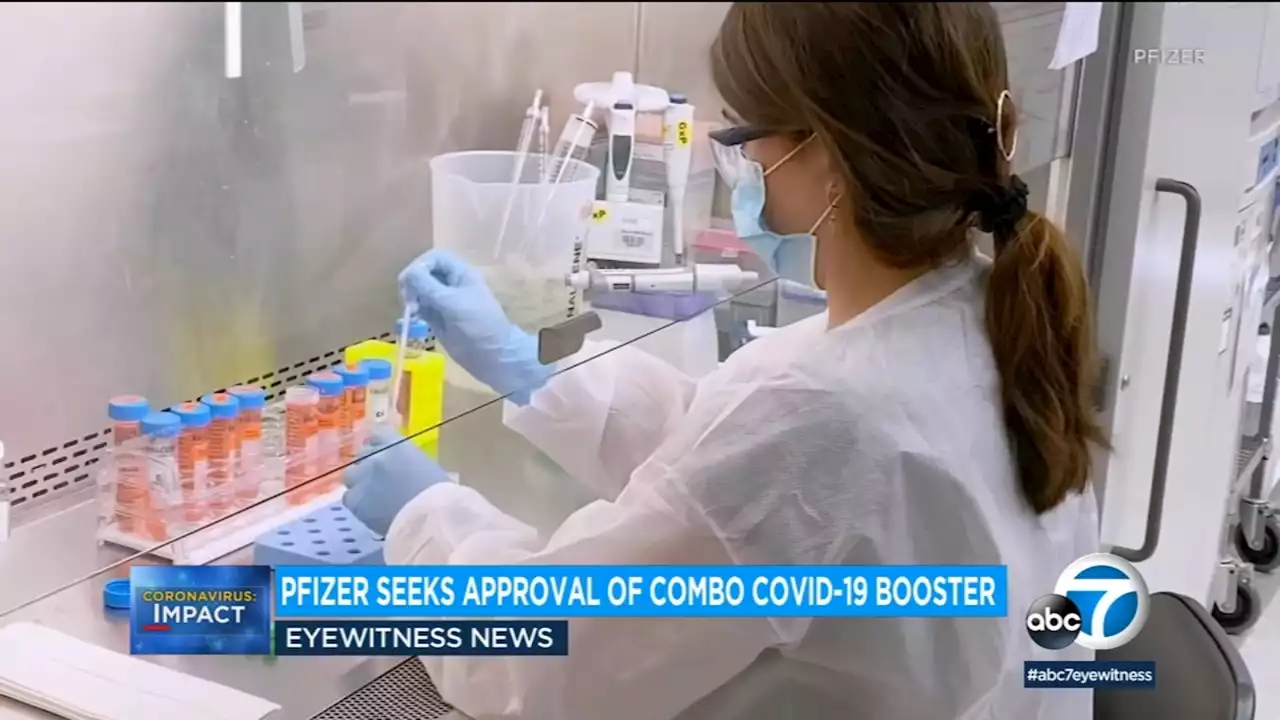 Pfizer asks FDA to authorize updated COVID vaccine adding protection from Omicron variantsThe FDA ordered vaccine makers to tweak their shots to target BA.4 and BA.5. Pfizer and its partner BioNTech aim to offer updated boosters to people 12 and older, and shots could begin within weeks if the FDA quickly clears the modified vaccine.
Pfizer asks FDA to authorize updated COVID vaccine adding protection from Omicron variantsThe FDA ordered vaccine makers to tweak their shots to target BA.4 and BA.5. Pfizer and its partner BioNTech aim to offer updated boosters to people 12 and older, and shots could begin within weeks if the FDA quickly clears the modified vaccine.
続きを読む »
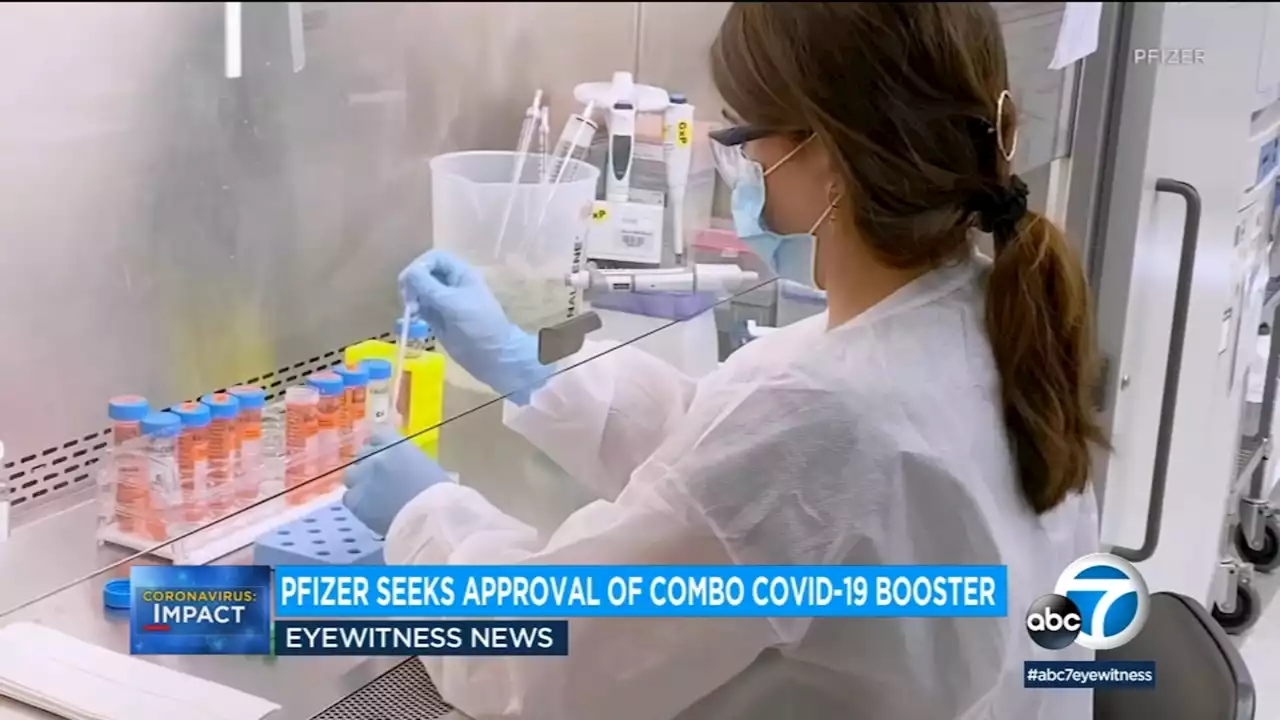 Pfizer asks FDA to authorize updated COVID vaccine adding protection from Omicron variantsThe FDA ordered vaccine makers to tweak their shots to target BA.4 and BA.5. Pfizer and its partner BioNTech aim to offer updated boosters to people 12 and older, and shots could begin within weeks if the FDA quickly clears the modified vaccine.
Pfizer asks FDA to authorize updated COVID vaccine adding protection from Omicron variantsThe FDA ordered vaccine makers to tweak their shots to target BA.4 and BA.5. Pfizer and its partner BioNTech aim to offer updated boosters to people 12 and older, and shots could begin within weeks if the FDA quickly clears the modified vaccine.
続きを読む »
 Pfizer-BioNTech submits new COVID vaccine booster targeting BA.5 to the FDA for authorizationPfizer and German partner BioNTech have submitted their new COVID-19 booster – that targets the omicron subvariant BA.5 – to the FDA for emergency use authorization.
Pfizer-BioNTech submits new COVID vaccine booster targeting BA.5 to the FDA for authorizationPfizer and German partner BioNTech have submitted their new COVID-19 booster – that targets the omicron subvariant BA.5 – to the FDA for emergency use authorization.
続きを読む »
 Pfizer Asks FDA To Greenlight New Omicron Booster Shots, Which Could Arrive This FallPfizer and BioNTech have asked the FDA to authorize an updated version of their COVID-19 vaccine for this fall — this one designed specifically to target the omicron subvariants that are dominant in the U.S.
Pfizer Asks FDA To Greenlight New Omicron Booster Shots, Which Could Arrive This FallPfizer and BioNTech have asked the FDA to authorize an updated version of their COVID-19 vaccine for this fall — this one designed specifically to target the omicron subvariants that are dominant in the U.S.
続きを読む »
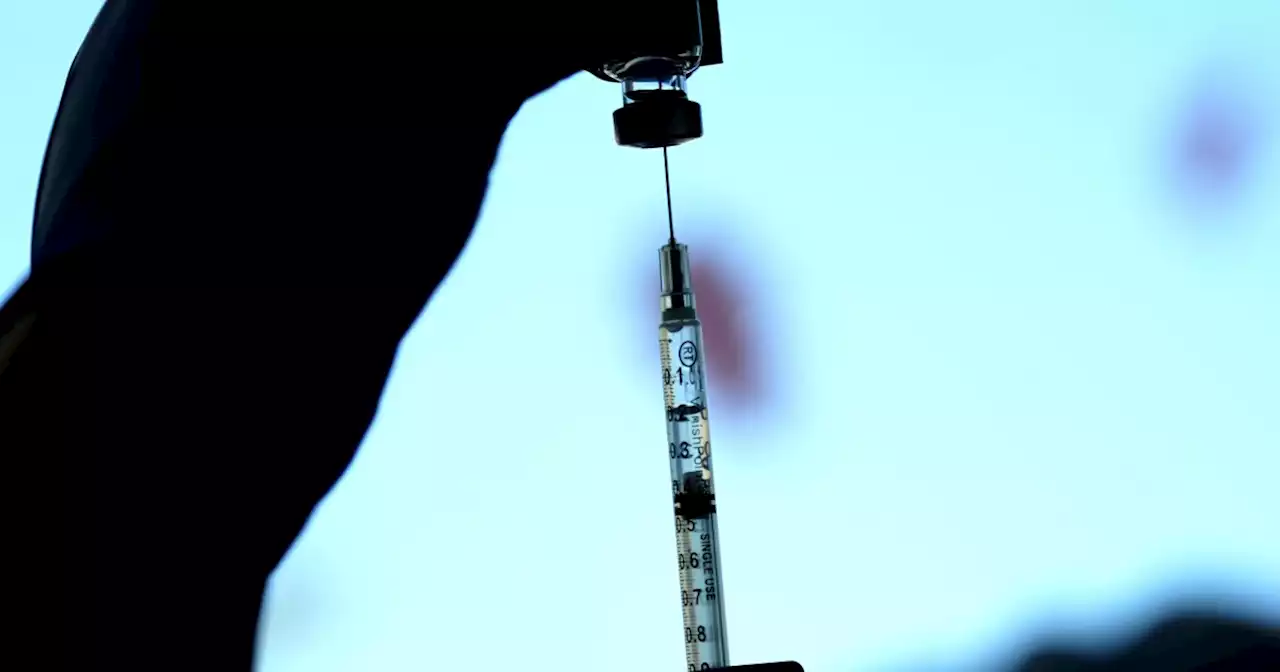 Pfizer asks FDA to greenlight new omicron booster shots, which could arrive this fallPfizer has submitted data on its bivalent COVID-19 booster shot that specifically targets the latest omicron subvariants. If authorized, the company says the shots could be ready as soon as September.
Pfizer asks FDA to greenlight new omicron booster shots, which could arrive this fallPfizer has submitted data on its bivalent COVID-19 booster shot that specifically targets the latest omicron subvariants. If authorized, the company says the shots could be ready as soon as September.
続きを読む »
 Pfizer asks FDA to greenlight new omicron booster shots, which could arrive this fallPfizer has submitted data on its bivalent COVID-19 booster shot that specifically targets the latest omicron subvariants. If authorized, the company says the shots could be ready as soon as September.
Pfizer asks FDA to greenlight new omicron booster shots, which could arrive this fallPfizer has submitted data on its bivalent COVID-19 booster shot that specifically targets the latest omicron subvariants. If authorized, the company says the shots could be ready as soon as September.
続きを読む »
