BREAKING FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11
The US Food and Drug Administration has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
"While it has largely been the case that COVID-19 tends to be less severe in children than adults, the omicron wave has seen more kids getting sick with the disease and being hospitalized, and children may also experience longer term effects, even following initially mild disease," FDA Commissioner Dr. Robert Califf said in a news release Tuesday.
Public health officials have urged Americans to stay up to date with their COVID-19 vaccinations, including all recommended booster doses, as the best way to protect themselves and the people around them.
日本 最新ニュース, 日本 見出し
Similar News:他のニュース ソースから収集した、これに似たニュース記事を読むこともできます。
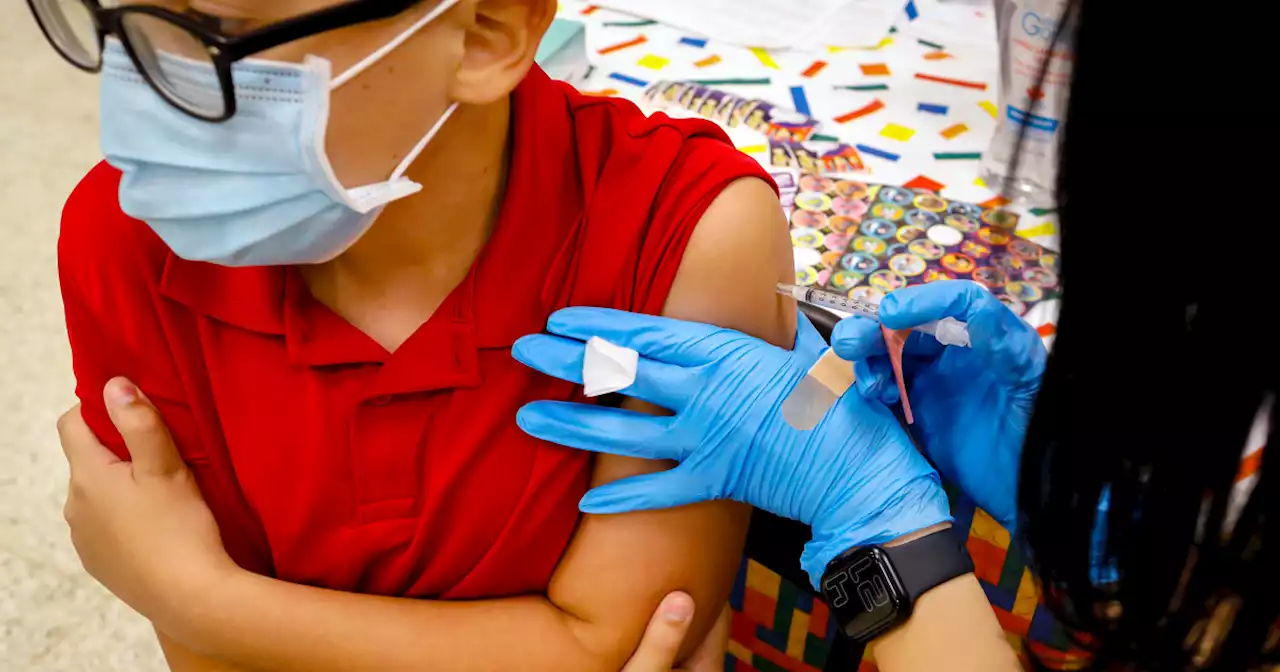 FDA authorizes Pfizer Covid booster for children 5 to 11 years oldA study in February found that two doses of the vaccine offered little protection against the omicron variant.
FDA authorizes Pfizer Covid booster for children 5 to 11 years oldA study in February found that two doses of the vaccine offered little protection against the omicron variant.
続きを読む »
 FDA Authorizes Pfizer Covid Booster Dose for Kids Ages 5 to 11 Years OldThe Food and Drug Administration on Tuesday authorized a Pfizer booster dose for children ages 5 through 11 years old at least five months after they complete their two-dose primary series. Dr. Peter Marks, head of the FDA division responsible for vaccines, said data increasingly shows that the protection provided by two shots wanes off over time. The FDA determined…
FDA Authorizes Pfizer Covid Booster Dose for Kids Ages 5 to 11 Years OldThe Food and Drug Administration on Tuesday authorized a Pfizer booster dose for children ages 5 through 11 years old at least five months after they complete their two-dose primary series. Dr. Peter Marks, head of the FDA division responsible for vaccines, said data increasingly shows that the protection provided by two shots wanes off over time. The FDA determined…
続きを読む »
 FDA authorizes first non-prescription test for COVID-19, flu, RSVNEW: The FDA has authorized the first non-prescription COVID-19 test that can also detect the flu and RSV.
FDA authorizes first non-prescription test for COVID-19, flu, RSVNEW: The FDA has authorized the first non-prescription COVID-19 test that can also detect the flu and RSV.
続きを読む »
 FDA Authorizes Nonprescription Test for Covid-19, Flu and RSVThe FDA authorized the first nonprescription test for Covid-19, influenza and RSV, in another step toward increasing patient access to tests for Covid-19 and other conditions
FDA Authorizes Nonprescription Test for Covid-19, Flu and RSVThe FDA authorized the first nonprescription test for Covid-19, influenza and RSV, in another step toward increasing patient access to tests for Covid-19 and other conditions
続きを読む »
 FDA: fluvoxamine doesn’t treat COVID-19 — and here’s 27 pages whyThat transparency isn’t normal.
FDA: fluvoxamine doesn’t treat COVID-19 — and here’s 27 pages whyThat transparency isn’t normal.
続きを読む »
 Without COVID-19 vaccines, death toll would be much higher: Pfizer analysisIn the wake of the tragic milestone of 1 million official COVID-19 deaths in the U.S., a new analysis shows that without vaccines, the virus would have likely claimed more than 100,000 additional lives in 2021.
Without COVID-19 vaccines, death toll would be much higher: Pfizer analysisIn the wake of the tragic milestone of 1 million official COVID-19 deaths in the U.S., a new analysis shows that without vaccines, the virus would have likely claimed more than 100,000 additional lives in 2021.
続きを読む »
