Named Lantidra, it's a cellular therapy developed using deceased donor pancreatic cells for the treatment of type 1 diabetes in adults.
“Severe hypoglycemia is a dangerous condition that can lead to injuries resulting from loss of consciousness or seizures,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research, in an
The findings reported that 21 of the study candidates were not required to use insulin for at least a year or longer. Additionally, eleven individuals did not use insulin for one to five years, while ten for more than five years.
日本 最新ニュース, 日本 見出し
Similar News:他のニュース ソースから収集した、これに似たニュース記事を読むこともできます。
 FDA OKs Pancreatic Islet Cell Therapy for Type 1 DiabetesApproval of Lantidra, a beta cell therapy derived from donor pancreatic islets, came despite transplant surgeons' concerns about FDA regulating these cells as biologics rather than transplanted organs.
FDA OKs Pancreatic Islet Cell Therapy for Type 1 DiabetesApproval of Lantidra, a beta cell therapy derived from donor pancreatic islets, came despite transplant surgeons' concerns about FDA regulating these cells as biologics rather than transplanted organs.
続きを読む »
 Gene therapy for severe hemophilia is approved by FDAU.S. health regulators have approved a gene therapy for the most common form of hemophilia
Gene therapy for severe hemophilia is approved by FDAU.S. health regulators have approved a gene therapy for the most common form of hemophilia
続きを読む »
 Merus stock jumps 10% after FDA’s action on cancer drugShares of Merus NV rallied as much as 10% in the extended session Thursday after the pharma company said the U.S. Food and Drug Administration has granted...
Merus stock jumps 10% after FDA’s action on cancer drugShares of Merus NV rallied as much as 10% in the extended session Thursday after the pharma company said the U.S. Food and Drug Administration has granted...
続きを読む »
 US FDA approves BioMarin's gene therapy for hemophilia AThe U.S. Food and Drug Administration on Thursday approved BioMarin Pharmaceutical's gene therapy for severe hemophilia A, the company said, giving patients with the inherited bleeding disorder an alternative to regular injections of missing blood proteins.
US FDA approves BioMarin's gene therapy for hemophilia AThe U.S. Food and Drug Administration on Thursday approved BioMarin Pharmaceutical's gene therapy for severe hemophilia A, the company said, giving patients with the inherited bleeding disorder an alternative to regular injections of missing blood proteins.
続きを読む »
 FDA approves BioMarin’s gene therapy drug for hemophiliaBioMarin Pharmaceutical Inc. received Food and Drug Administration approval of its gene therapy Roctavian to treat hemophilia A, the agency said late...
FDA approves BioMarin’s gene therapy drug for hemophiliaBioMarin Pharmaceutical Inc. received Food and Drug Administration approval of its gene therapy Roctavian to treat hemophilia A, the agency said late...
続きを読む »
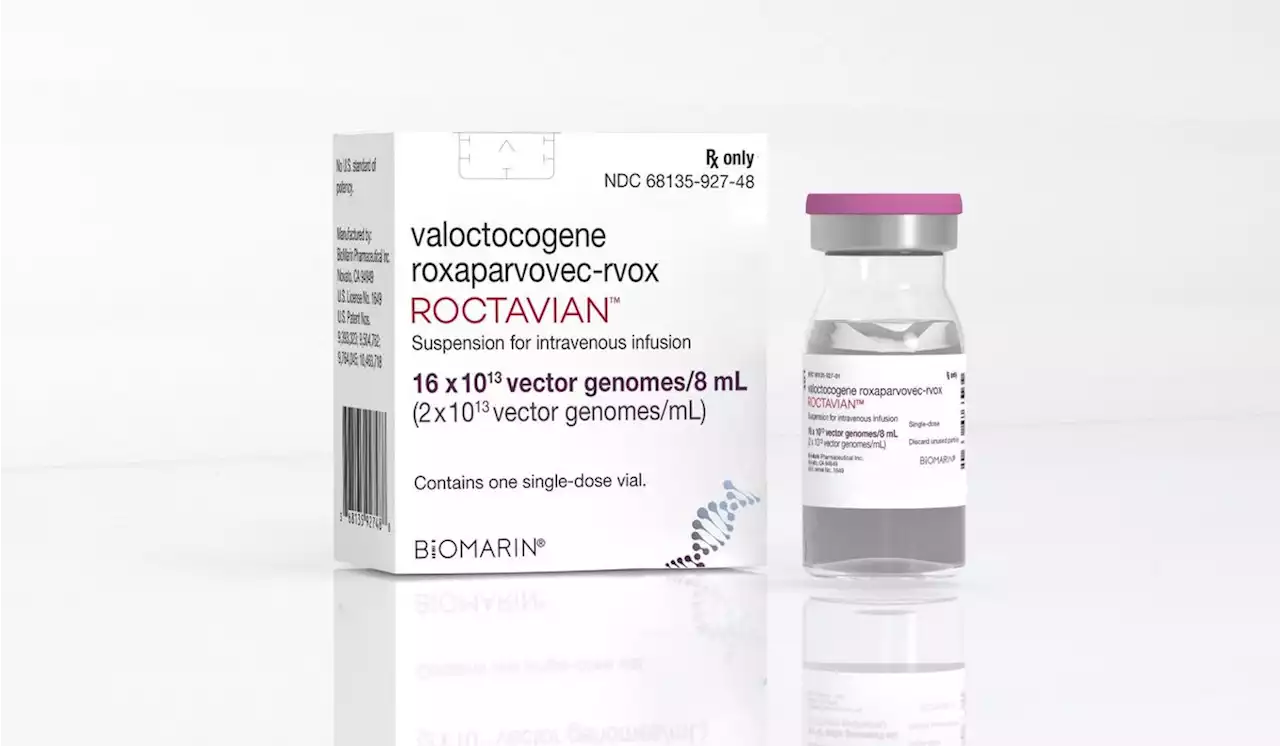 Gene therapy for severe hemophilia is approved by FDAU.S. officials on Thursday approved drugmaker BioMarin's gene therapy for the most common form of hemophilia, an infused treatment that can significantly reduce dangerous bleeding problems.
Gene therapy for severe hemophilia is approved by FDAU.S. officials on Thursday approved drugmaker BioMarin's gene therapy for the most common form of hemophilia, an infused treatment that can significantly reduce dangerous bleeding problems.
続きを読む »
