The U.S. Food and Drug Administration on Wednesday approved selling naloxone without a prescription
, setting the overdose-reversing drug on course to become the first opioid treatment drug to be sold over the counter.
Making naloxone available more widely is seen as a key strategy to control the nationwide overdose crisis, which has been linked to more than 100,000 U.S. deaths a year. The majority of those deaths are tied to opioids, primarily potent synthetic versions such as fentanyl that can take multiple doses of naloxone to reverse.
The nonprofit Harm Reduction Therapeutics Inc., which has funding from OxyContin maker Purdue Pharma, has an application before the FDA to distribute its version of spray naloxone without a prescription.Even before the FDA's action, pharmacies could sell naloxone without a prescription because officials in every state have allowed it.
Now, he said, some people are concerned about getting naloxone at pharmacies because their insurers will know they're getting it.
日本 最新ニュース, 日本 見出し
Similar News:他のニュース ソースから収集した、これに似たニュース記事を読むこともできます。
 FDA approves over-the-counter Narcan. Here’s what it meansThe U.S. Food and Drug Administration on Wednesday approved selling naloxone without a prescription, setting the overdose-reversing drug on course...
FDA approves over-the-counter Narcan. Here’s what it meansThe U.S. Food and Drug Administration on Wednesday approved selling naloxone without a prescription, setting the overdose-reversing drug on course...
続きを読む »
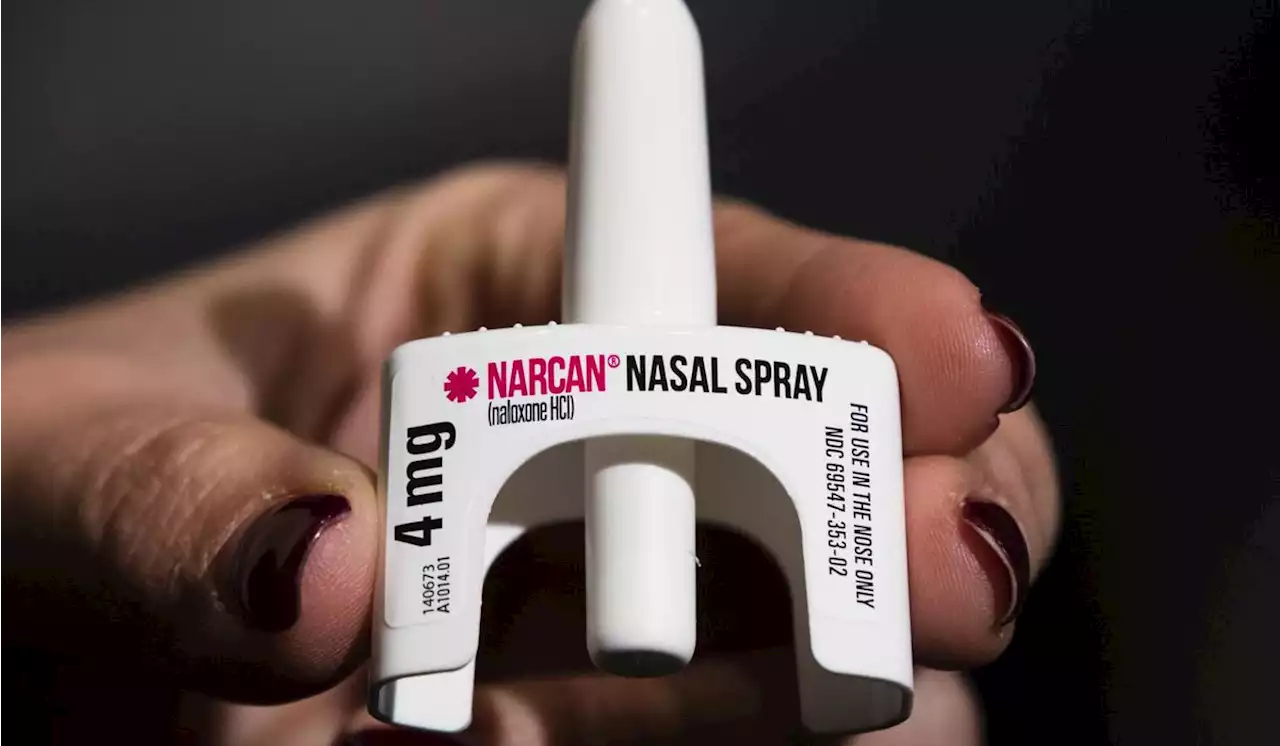 FDA approves over-the-counter Narcan; here’s what it meansThe U.S. Food and Drug Administration on Wednesday approved selling naloxone without a prescription, setting the overdose-reversing drug on course to become the first opioid treatment drug to be sold over the counter.
FDA approves over-the-counter Narcan; here’s what it meansThe U.S. Food and Drug Administration on Wednesday approved selling naloxone without a prescription, setting the overdose-reversing drug on course to become the first opioid treatment drug to be sold over the counter.
続きを読む »
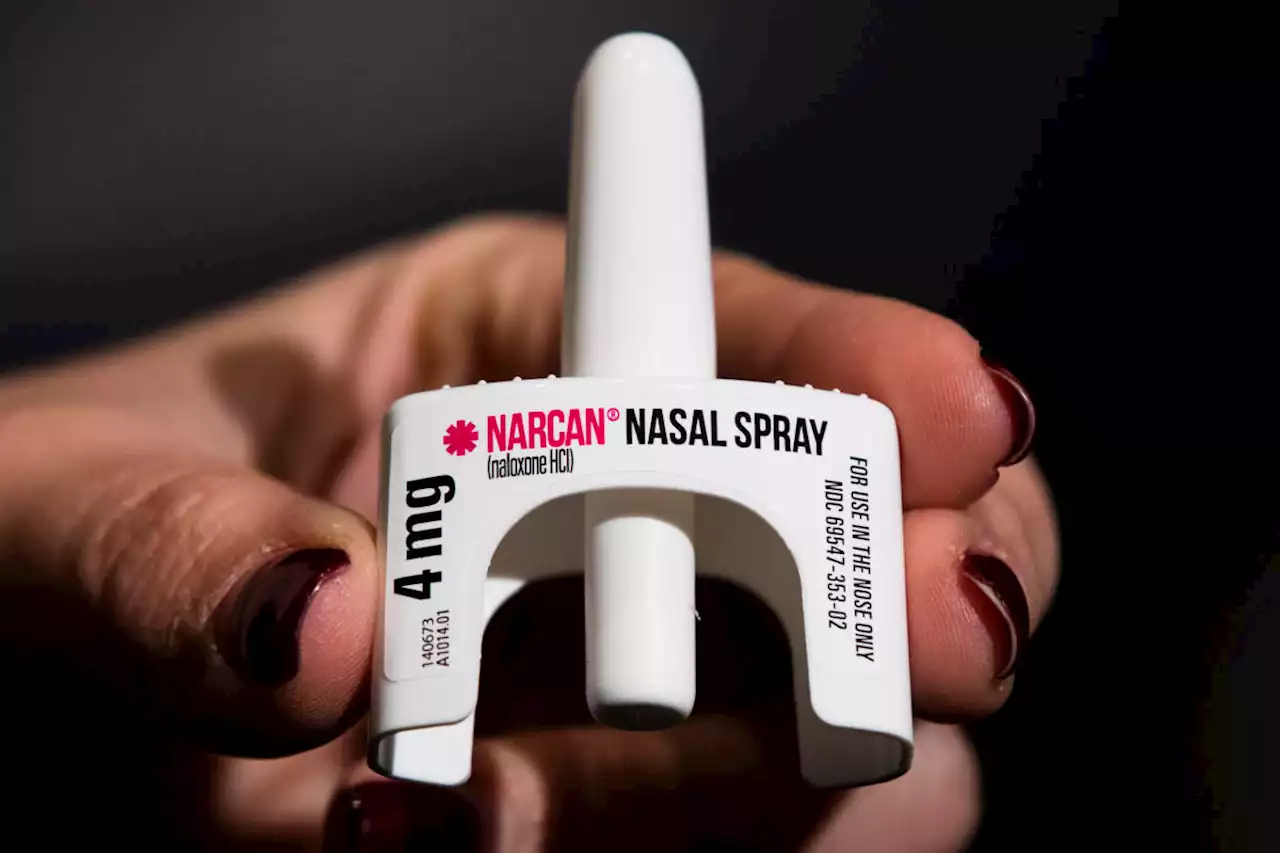 FDA approves over-the-counter Narcan. Here's what it meansThe U.S. Food and Drug Administration on Wednesday approved selling naloxone without a prescription, setting the overdose-reversing drug on course to become the first opioid treatment drug to be sold over the counter. WHAT IS NARCAN? The approved branded nasal spray from Gaithersburg, Maryland-based Emergent BioSolutions is the best-known form of naloxone.
FDA approves over-the-counter Narcan. Here's what it meansThe U.S. Food and Drug Administration on Wednesday approved selling naloxone without a prescription, setting the overdose-reversing drug on course to become the first opioid treatment drug to be sold over the counter. WHAT IS NARCAN? The approved branded nasal spray from Gaithersburg, Maryland-based Emergent BioSolutions is the best-known form of naloxone.
続きを読む »
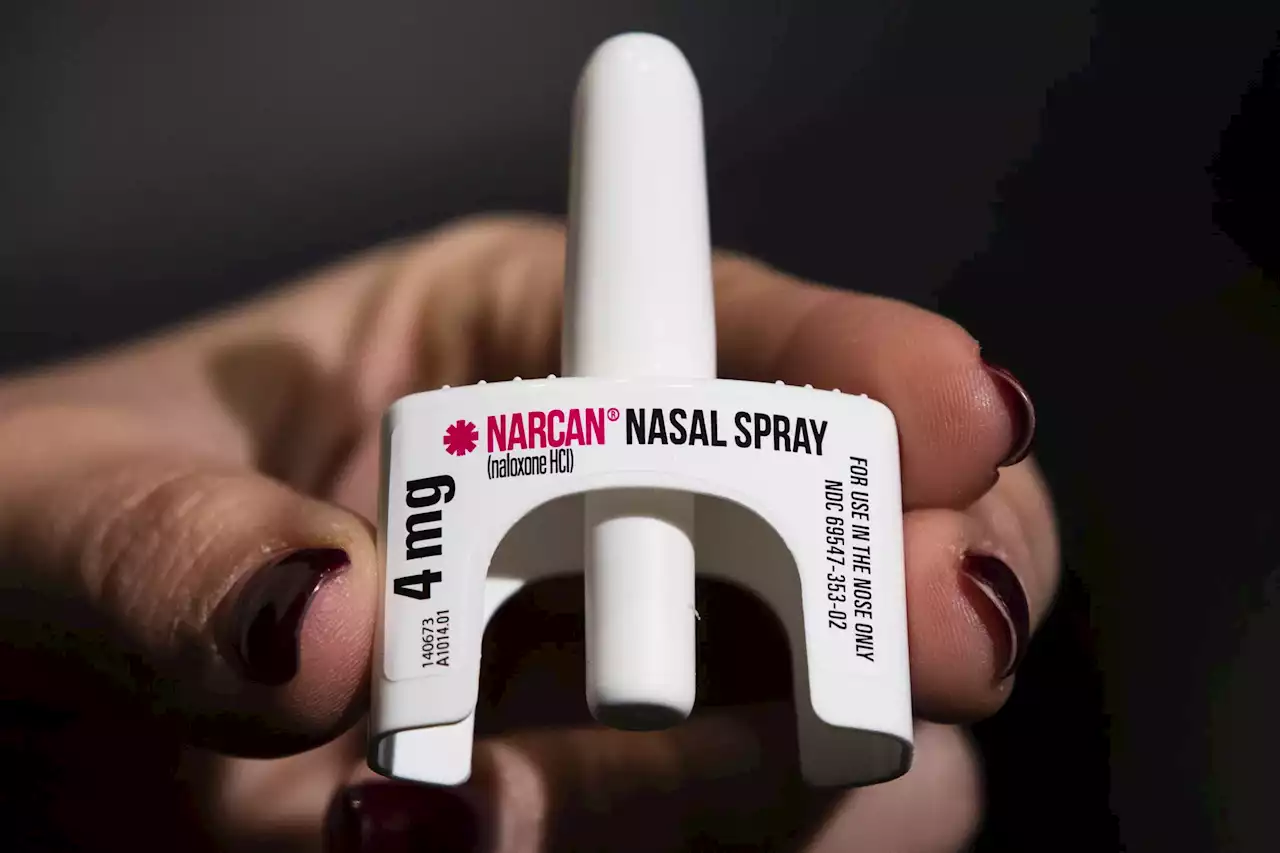 FDA approves over-the-counter Narcan. Here's what it meansThe U.S. Food and Drug Administration approved selling naloxone without a prescription, setting the overdose-reversing drug on course to become the first opioid treatment drug to be sold over the counter. Here's what it means.
FDA approves over-the-counter Narcan. Here's what it meansThe U.S. Food and Drug Administration approved selling naloxone without a prescription, setting the overdose-reversing drug on course to become the first opioid treatment drug to be sold over the counter. Here's what it means.
続きを読む »
 FDA proposes overhaul of fast-track process for cancer medsThe Food and Drug Administration is eyeing policy changes that could make drugmakers conduct more stringent trials to win fast-track approvals of cancer drugs.
FDA proposes overhaul of fast-track process for cancer medsThe Food and Drug Administration is eyeing policy changes that could make drugmakers conduct more stringent trials to win fast-track approvals of cancer drugs.
続きを読む »
