The drug — part of a class of medicines known as JAK inhibitors — is the first approved treatment for alopecia areata.
The Food and Drug Administration approved baricitinib, a once-a-day pill developed by the drugmaker Eli Lilly to treat alopecia areata, an autoimmune disease that triggers sudden hair loss.People suffering from a rare autoimmune disorder that causes their hair to suddenly fall out, often in clumps, now have a treatment option for the first time.baricitinib, a once-a-day pill developed by the drugmaker Eli Lilly to treat alopecia areata, an autoimmune disease that triggers sudden hair loss.
People with the autoimmune disease can experience hair loss anywhere on their body, including around the scalp, eyebrows and eyelashes, according to the , involving a total of 1,200 patients with severe alopecia areata, found that about 40 percent of people who received a daily 4-milligram dose of the drug regrew all or almost all of their hair after 36 weeks.That compares to the about 20 percent of people who received a lower 2-milligram dose of the drug and less than 6 percent of the people in the placebo group.The drug is a breakthrough for people with the autoimmune disorder, said Dr.
Dr. Luis Garza, a dermatologist at the Johns Hopkins University School of Medicine who treats people with alopecia, said he's had patients quit their jobs or avoid going out in public altogether over the stress of losing their hair.Up until now, there weren't many options for people with the condition: They often relied on steroid injections or unproven creams in an attempt to reverse the condition, Garza, who researches hair loss, said.
日本 最新ニュース, 日本 見出し
Similar News:他のニュース ソースから収集した、これに似たニュース記事を読むこともできます。
 FDA Approves First Drug to Treat Hair Loss Caused by Severe AlopeciaThe FDA on Monday approved the first-ever treatment for alopecia areata, an autoimmune disorder that causes hair loss and was thrown into the spotlight after actor Will Smith slapped comedian Chris Rock at the Oscars.
FDA Approves First Drug to Treat Hair Loss Caused by Severe AlopeciaThe FDA on Monday approved the first-ever treatment for alopecia areata, an autoimmune disorder that causes hair loss and was thrown into the spotlight after actor Will Smith slapped comedian Chris Rock at the Oscars.
続きを読む »
 The FDA Just Approved The First Oral Tablet For Treating Severe Alopecia AreataThe Food and Drug Administration on Monday approved a drug called baricitinib as the first oral tablet for treating severe alopecia areata, an autoimmune disorder affecting more than 300,000 people in the United States every year.
The FDA Just Approved The First Oral Tablet For Treating Severe Alopecia AreataThe Food and Drug Administration on Monday approved a drug called baricitinib as the first oral tablet for treating severe alopecia areata, an autoimmune disorder affecting more than 300,000 people in the United States every year.
続きを読む »
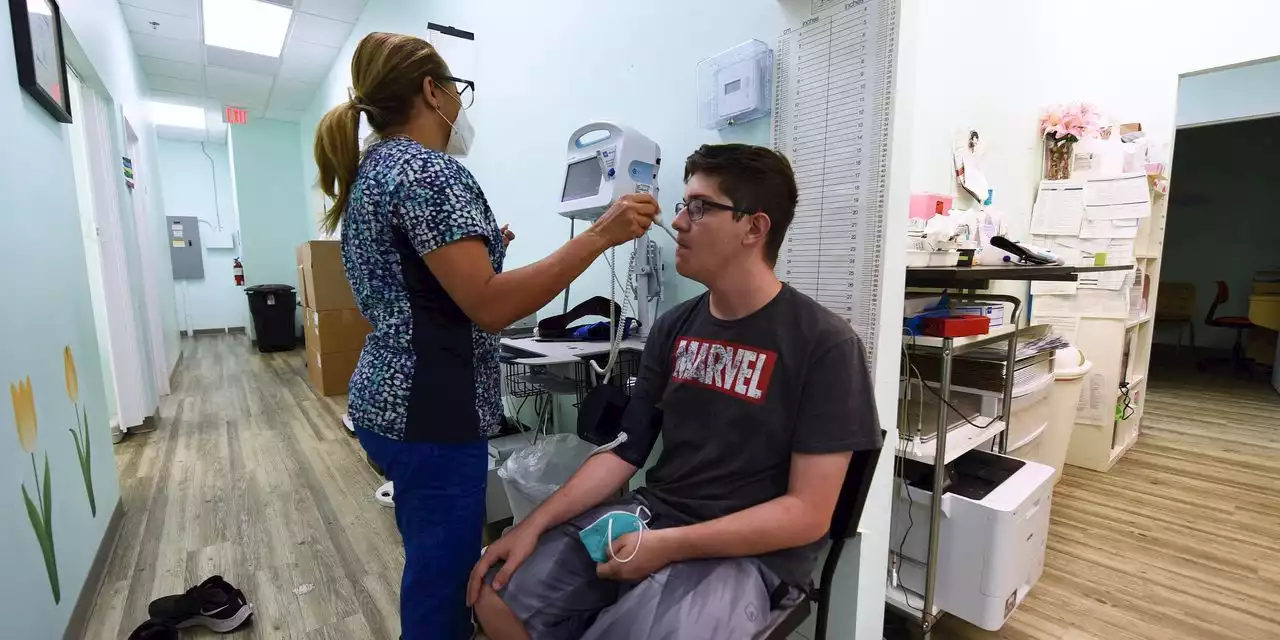 FDA Advisers Considering Moderna’s Covid-19 Vaccine for Ages 6 to 17An advisory committee is set to vote Tuesday on whether to recommend that the Food and Drug Administration authorize use of the Moderna vaccine for 6- to 17-year-olds.
FDA Advisers Considering Moderna’s Covid-19 Vaccine for Ages 6 to 17An advisory committee is set to vote Tuesday on whether to recommend that the Food and Drug Administration authorize use of the Moderna vaccine for 6- to 17-year-olds.
続きを読む »
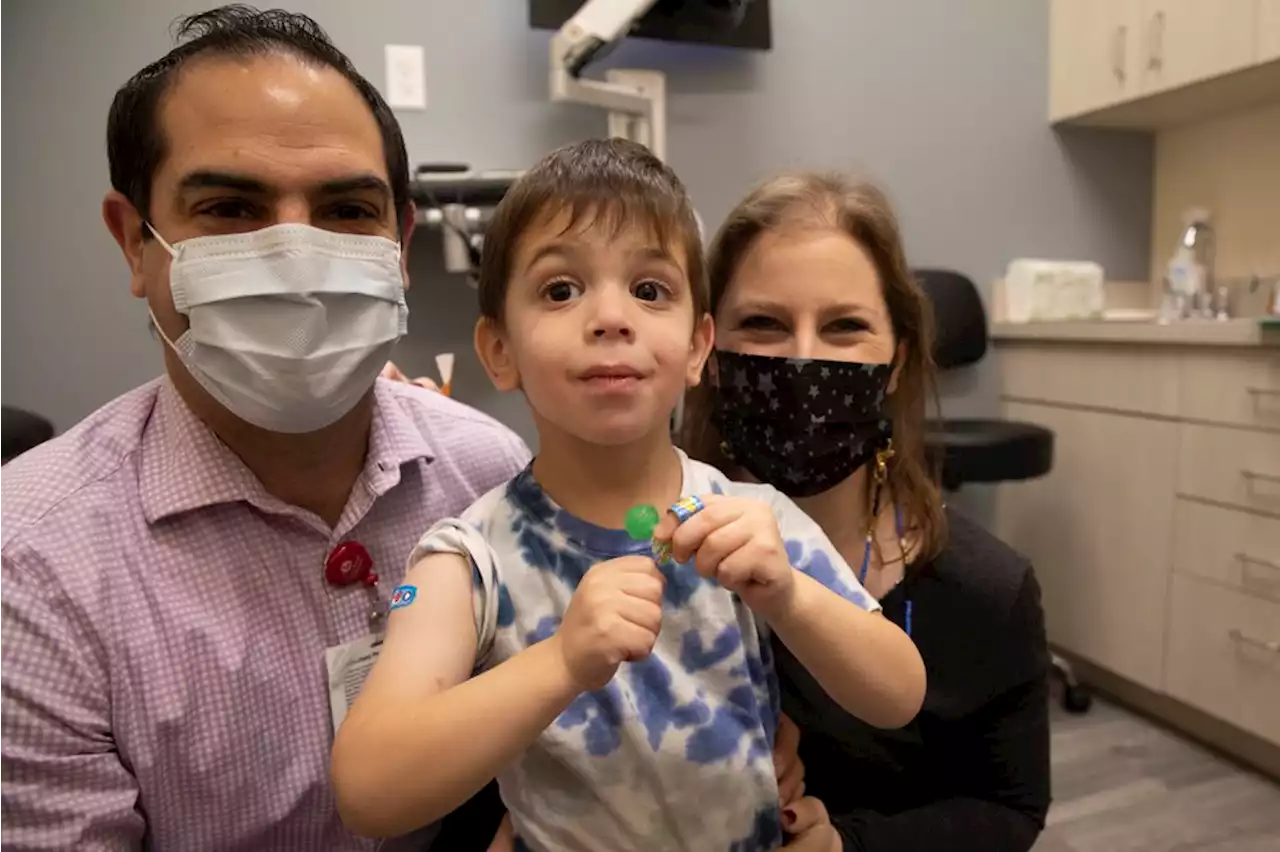 FDA advisers consider Moderna’s COVID shots for teens, younger kidsFDA scientists said there were no confirmed cases of the heart inflammation in Moderna’s kid studies.
FDA advisers consider Moderna’s COVID shots for teens, younger kidsFDA scientists said there were no confirmed cases of the heart inflammation in Moderna’s kid studies.
続きを読む »
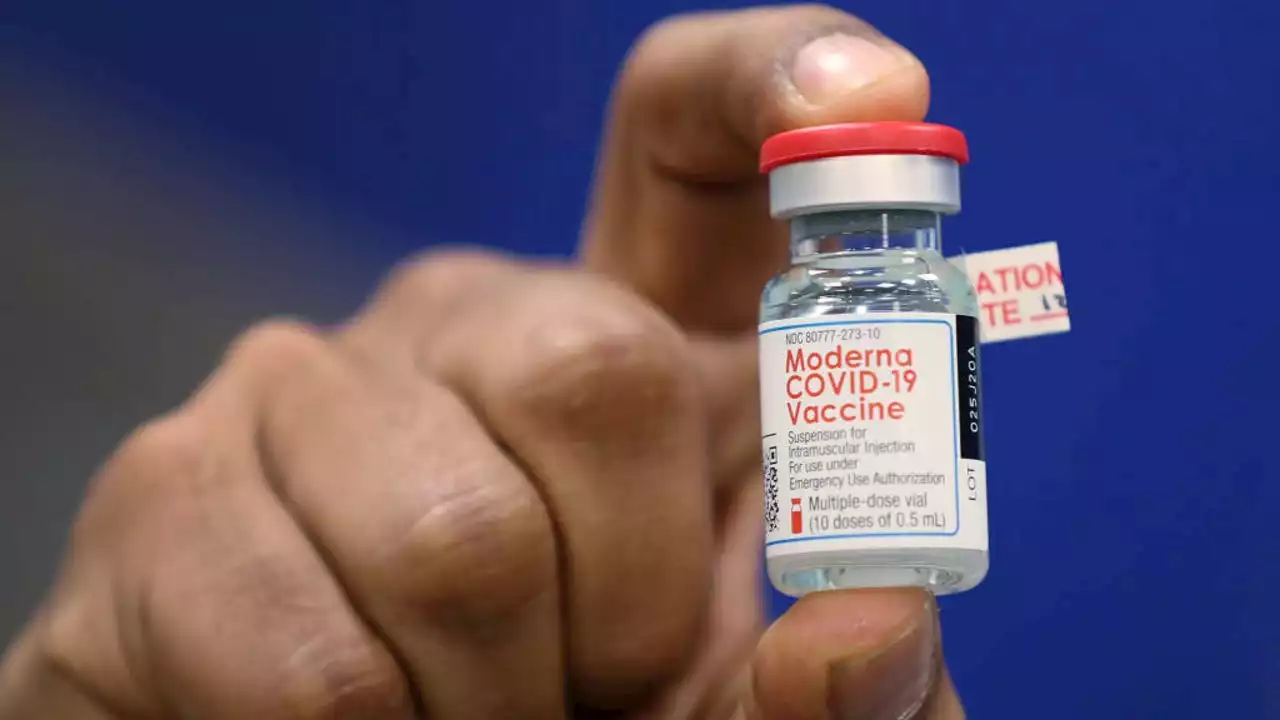 FDA panel endorses Moderna's COVID vaccine for teens, school-age kidsAn FDA advisory panel endorsed on Tuesday Moderna's COVID-19 vaccine for teenagers and school-aged children.
FDA panel endorses Moderna's COVID vaccine for teens, school-age kidsAn FDA advisory panel endorsed on Tuesday Moderna's COVID-19 vaccine for teenagers and school-aged children.
続きを読む »
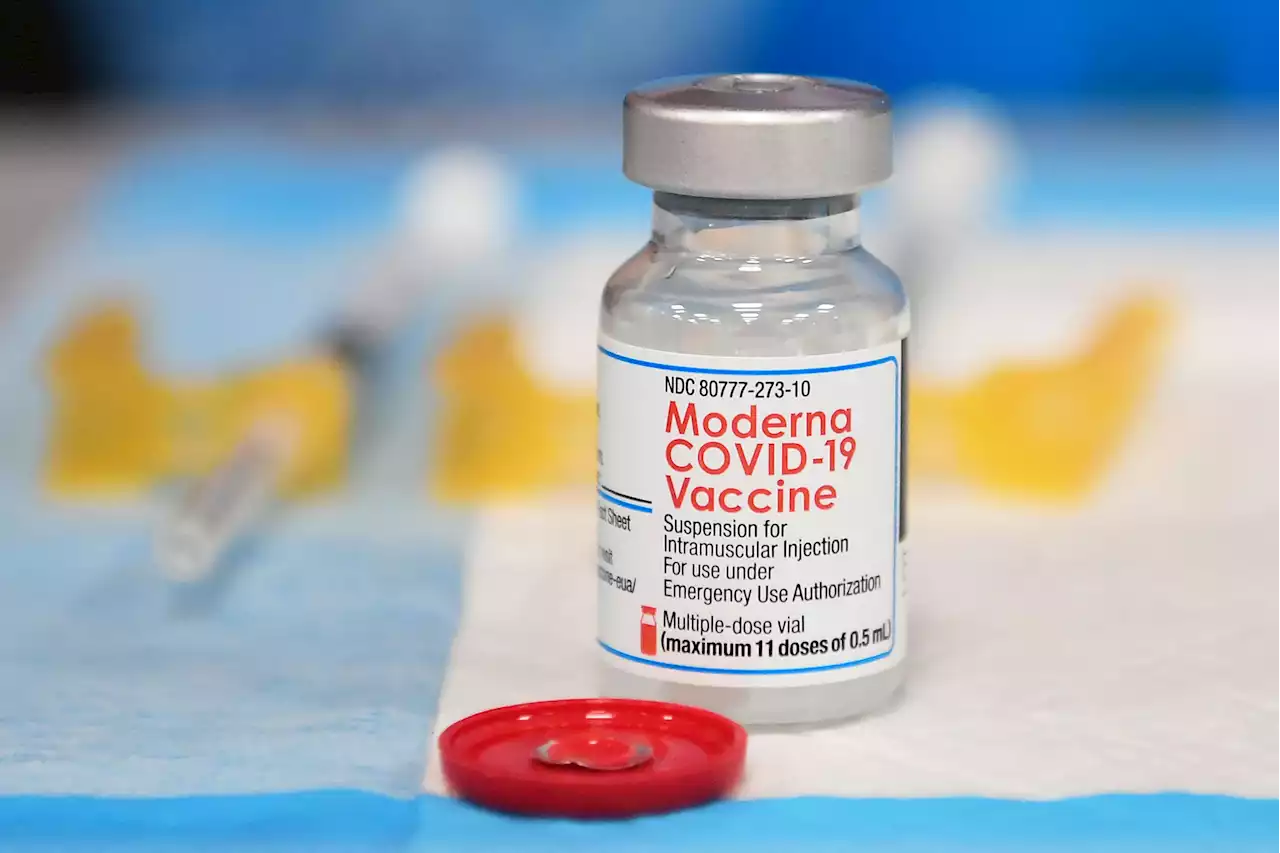 FDA Advisers Back Moderna's COVID-19 Vaccine for Older KidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens. The Food and Drug Administration’s outside experts voted unanimously that Moderna’s vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer’s vaccine. The same FDA…
FDA Advisers Back Moderna's COVID-19 Vaccine for Older KidsA government advisory panel Tuesday endorsed a second brand of COVID-19 vaccine for school-age children and teens. The Food and Drug Administration’s outside experts voted unanimously that Moderna’s vaccine is safe and effective enough to give kids ages 6 to 17. If the FDA agrees, it would become the second option for those children, joining Pfizer’s vaccine. The same FDA…
続きを読む »
