FDA advisers will meet Thursday to discuss how the next round of Covid boosters should be updated to target strains that may be circulating this fall.
and XBB.1.9.1, which make up about 18% and 12% of all new cases, respectively. The XBB strains haven’t caused a surge in cases as much as previous variants.
“These data suggest that an updated strain composition of Covid-19 vaccines to more closely match currently circulating Omicron sublineages is warranted for the 2023–2024 vaccination campaign,” the scientists wrote. It’s unclear if the new vaccines will be recommended for everyone this fall. A CDC advisory committee, called Advisory Committee on Immunization Practices, is scheduled to meet next week to talk about updated Covid boosters, among other vaccines.
“In my mind, it seems like a logical framework,” said Levy, who is a member of the FDA’s advisory committee and will be involved in Thursday’s meeting. “It will boost your antibody response and it probably affects your T cells as well, in a positive way.”
日本 最新ニュース, 日本 見出し
Similar News:他のニュース ソースから収集した、これに似たニュース記事を読むこともできます。
 Updated Covid vaccines need to target XBB omicron variants this fall, FDA staff saysVaccine manufacturers Pfizer, Moderna and Novavax will be expected to update their shots according to the strain the FDA selects for the fall.
Updated Covid vaccines need to target XBB omicron variants this fall, FDA staff saysVaccine manufacturers Pfizer, Moderna and Novavax will be expected to update their shots according to the strain the FDA selects for the fall.
続きを読む »
 COVID shots should target XBB variants in 2023-24 campaign, US FDA staff sayCOVID-19 vaccines being developed and manufactured for the 2023-2024 campaign should target one of the currently dominant XBB variants, the U.S. Food and Drug Administration's (FDA) staff reviewers said on Monday.
COVID shots should target XBB variants in 2023-24 campaign, US FDA staff sayCOVID-19 vaccines being developed and manufactured for the 2023-2024 campaign should target one of the currently dominant XBB variants, the U.S. Food and Drug Administration's (FDA) staff reviewers said on Monday.
続きを読む »
 FDA Panel Unanimously Endorses Lecanemab for Alzheimer'sFDA advisory committee determines phase 3 study of lecanemab, a monoclonal antibody directed against amyloid beta confirms the drug's clinical benefit for patients with Alzheimer's disease.
FDA Panel Unanimously Endorses Lecanemab for Alzheimer'sFDA advisory committee determines phase 3 study of lecanemab, a monoclonal antibody directed against amyloid beta confirms the drug's clinical benefit for patients with Alzheimer's disease.
続きを読む »
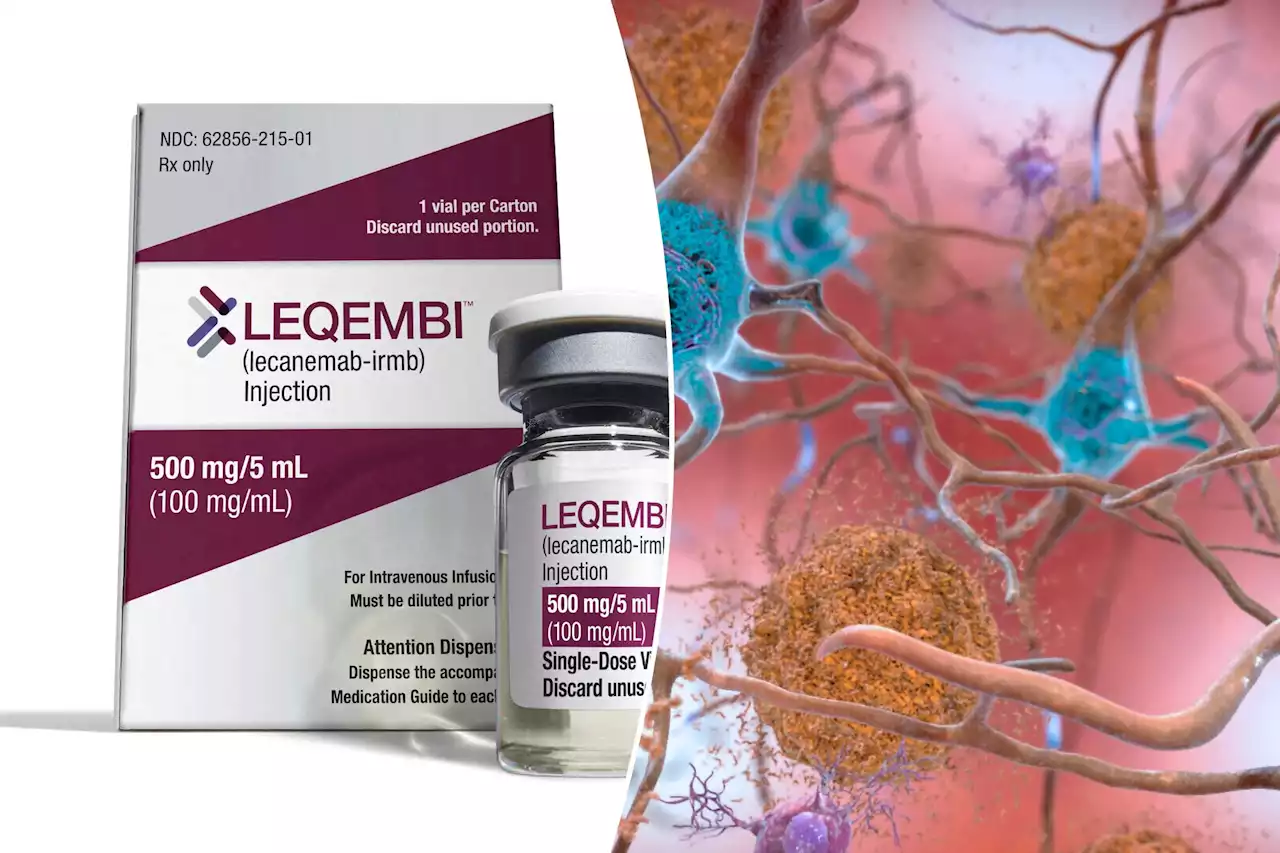 FDA approves ‘robust’ dementia drug that slows cognitive declineLecanemab, marketed as Leqembi, could be a game-changer for people with Alzheimer’s disease — but only if they can get it.
FDA approves ‘robust’ dementia drug that slows cognitive declineLecanemab, marketed as Leqembi, could be a game-changer for people with Alzheimer’s disease — but only if they can get it.
続きを読む »
 FDA Approves First Treatment for Constipation in KidsThe FDA has expanded the indication for linaclotide to children as young as age 6 years with functional constipation, making it the first approved treatment for pediatric functional constipation.
FDA Approves First Treatment for Constipation in KidsThe FDA has expanded the indication for linaclotide to children as young as age 6 years with functional constipation, making it the first approved treatment for pediatric functional constipation.
続きを読む »
 FDA Approves First-Ever OTC Erectile Dysfunction GelA topical gel that may work faster than erectile dysfunction pills has been approved for over-the-counter use in the United States. The gel, which can help users get an erection within 10 minutes, is already available without a prescription in Europe.
FDA Approves First-Ever OTC Erectile Dysfunction GelA topical gel that may work faster than erectile dysfunction pills has been approved for over-the-counter use in the United States. The gel, which can help users get an erection within 10 minutes, is already available without a prescription in Europe.
続きを読む »
