The FDA failed to adhere to its own guidance and internal practices during the approval process for Biogen's Alzheimer's drug Aduhelm, which was 'rife with irregularities,' a congressional report showed.
WASHINGTON - The U.S. Food and Drug Administration failed to adhere to its own guidance and internal practices during the approval process for Biogen's Alzheimer's drug Aduhelm, which was "rife with irregularities," a congressional report showed on Thursday.
It was authorized based on evidence that it could reduce brain plaques, a likely contributor to Alzheimer's, rather than proof that it slowed progression of the lethal mind-wasting disease. "The findings in this report raise serious concerns about FDA's lapses in protocol and Biogen's disregard of efficacy and access in the approval process for Aduhelm," the report, prepared by the staffs of the House Committee on Oversight and Reform and House Committee on Energy and Commerce, concluded.
"As stated in the congressional report, an review concluded that, 'There is no evidence that these interactions with the sponsor in advance of filing were anything but appropriate in this situation,'" Biogen said. The FDA inappropriately collaborated with Biogen on a joint briefing document for the Peripheral and Central Nervous System Advisory Committee, the report said, with FDA and Biogen staff working closely for months ahead of the Nov. 6, 2020 meeting to prepare the document, which failed to adequately represent differing views within the agency.
日本 最新ニュース, 日本 見出し
Similar News:他のニュース ソースから収集した、これに似たニュース記事を読むこともできます。
 FDA Faulted for Working Improperly With Biogen Before Clearing Alzheimer’s DrugBreaking: The FDA inappropriately collaborated with Biogen before approving its Alzheimer's drug Aduhelm, a report by House Democrats found
FDA Faulted for Working Improperly With Biogen Before Clearing Alzheimer’s DrugBreaking: The FDA inappropriately collaborated with Biogen before approving its Alzheimer's drug Aduhelm, a report by House Democrats found
続きを読む »
 Congressional report: U.S. FDA broke own protocols in approving Biogen Alzheimer's drugThe U.S. Food and Drug Administration failed to adhere to its own guidance and internal practices during the approval process for Biogen's Alzheimer's drug Aduhelm, which was 'rife with irregularities,' a congressional report showed on Thursday.
Congressional report: U.S. FDA broke own protocols in approving Biogen Alzheimer's drugThe U.S. Food and Drug Administration failed to adhere to its own guidance and internal practices during the approval process for Biogen's Alzheimer's drug Aduhelm, which was 'rife with irregularities,' a congressional report showed on Thursday.
続きを読む »
 Congressional probe faults FDA on Biogen Alzheimer's drug approvalAn 18-month congressional investigation found the FDA didn't follow its own guidance and inappropriately collaborated with drugmaker Biogen before the approval of a controversial Alzheimer's drug last year.
Congressional probe faults FDA on Biogen Alzheimer's drug approvalAn 18-month congressional investigation found the FDA didn't follow its own guidance and inappropriately collaborated with drugmaker Biogen before the approval of a controversial Alzheimer's drug last year.
続きを読む »
 FDA Fast-Tracks Approval of Overdose Reversal Drug for OTC UseAn inexpensive overdose-reversal drug in nasal spray form is being fast-tracked by the FDA for consideration to be sold over-the-counter.
FDA Fast-Tracks Approval of Overdose Reversal Drug for OTC UseAn inexpensive overdose-reversal drug in nasal spray form is being fast-tracked by the FDA for consideration to be sold over-the-counter.
続きを読む »
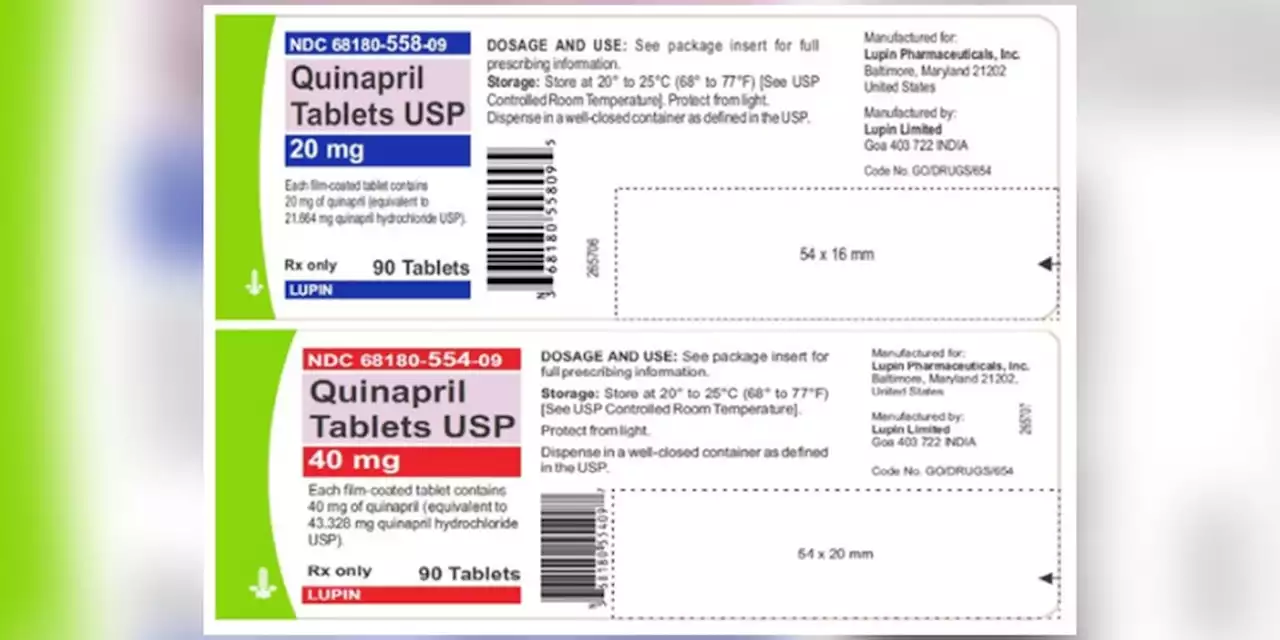 Blood pressure tablets recalled due to potential cancer risks, FDA saysQuinaprill tablets are used to treat hypertension and are distributed across the United States to wholesalers, pharmacies and supermarkets.
Blood pressure tablets recalled due to potential cancer risks, FDA saysQuinaprill tablets are used to treat hypertension and are distributed across the United States to wholesalers, pharmacies and supermarkets.
続きを読む »
