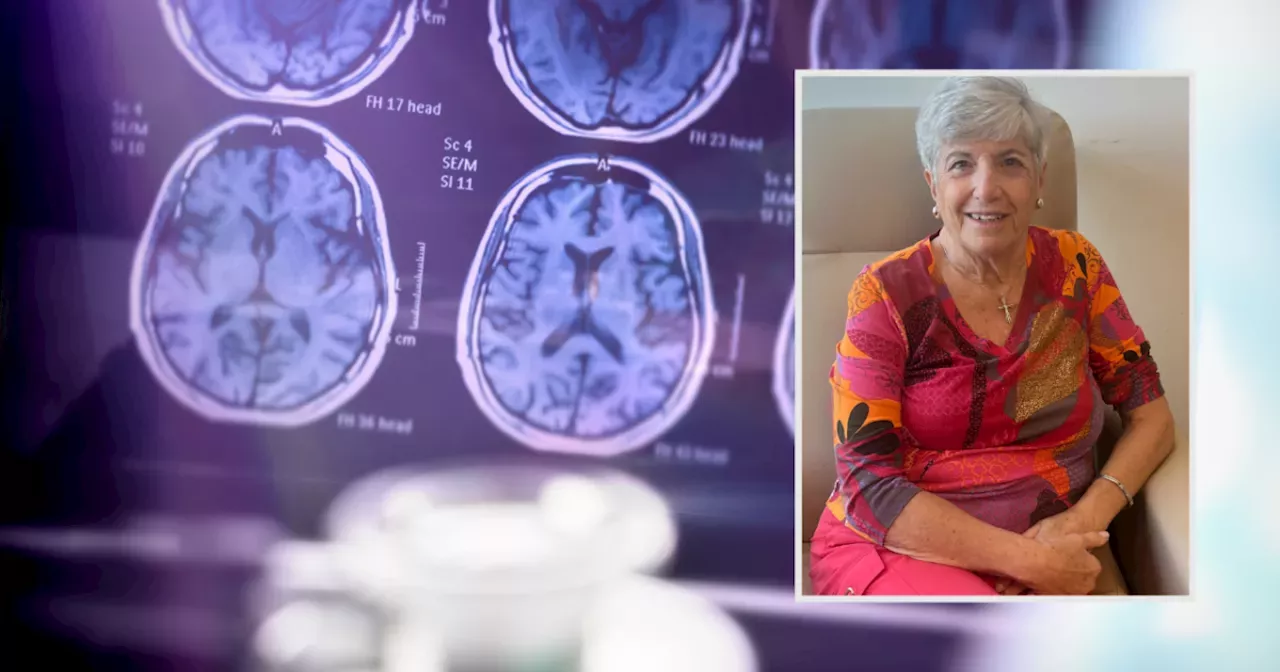
'It gives people like me so much hope.' The FDA approved an Eli Lilly drug for people with early signs of Alzheimer’s.
INDIANAPOLIS — A newly approved drug is a sign of hope for thousands of Hoosiers facing Alzheimer’s disease and the loved ones who care for them.
"I'm just, I feel overwhelmed. I've been very emotional today. Because now there's so much hope for other people with the same diagnosis that I have," Patricia Bishara said."When I heard the diagnosis, my first thoughts were, I won't remember my children and my grandchildren's names in five years," Bishara said.
"The new drug is called Kisunla. And it can slow the progression of Alzheimer's disease again when it's provided to those living in the early stages of the disease," Natalie Sutton, Chapter Executive of Alzheimer’s Association Greater Indiana Chapter said.
日本 最新ニュース, 日本 見出し
Similar News:他のニュース ソースから収集した、これに似たニュース記事を読むこともできます。
 Alzheimer’s Drug with Modest Benefits Gets Green Light from FDA AdvisersThe drug donanemab slows progression of symptoms in people with early stages of Alzheimer’s disease, but questions linger about the durability of its effect
Alzheimer’s Drug with Modest Benefits Gets Green Light from FDA AdvisersThe drug donanemab slows progression of symptoms in people with early stages of Alzheimer’s disease, but questions linger about the durability of its effect
続きを読む »
 AFA responds to approval of Alzheimer’s Drug by FDA Advisory CommitteeThis new drug works by slowing the progression of the disease when administered within the early stages of Alzheimer’s.
AFA responds to approval of Alzheimer’s Drug by FDA Advisory CommitteeThis new drug works by slowing the progression of the disease when administered within the early stages of Alzheimer’s.
続きを読む »
 Independent FDA Panel Endorses New Alzheimer’s Disease TreatmentAn FDA advisory panel unanimously gave a green light on Monday to the Alzheimer’s disease treatment donanemab for use during early stages of the disease.
Independent FDA Panel Endorses New Alzheimer’s Disease TreatmentAn FDA advisory panel unanimously gave a green light on Monday to the Alzheimer’s disease treatment donanemab for use during early stages of the disease.
続きを読む »
 Experimental Alzheimer’s drug gets FDA advisory panel's thumbs-up: ‘Progress is happening’An experimental Alzheimer’s drug, donanemab, was endorsed by a U.S. Food and Drug Administration advisory panel on Monday. Industry experts and doctors shared reactions.
Experimental Alzheimer’s drug gets FDA advisory panel's thumbs-up: ‘Progress is happening’An experimental Alzheimer’s drug, donanemab, was endorsed by a U.S. Food and Drug Administration advisory panel on Monday. Industry experts and doctors shared reactions.
続きを読む »
 FDA panel backs Alzheimer's drug that can reportedly slow cognitive declineRooted in fact-based, transparent reporting, Newsy is an award-winning opinion-free network owned by the E.W. Scripps Company that is relentlessly focused on “the why” of every story and seeks to enable a more intimate and immersive understanding of the issues that matter.
FDA panel backs Alzheimer's drug that can reportedly slow cognitive declineRooted in fact-based, transparent reporting, Newsy is an award-winning opinion-free network owned by the E.W. Scripps Company that is relentlessly focused on “the why” of every story and seeks to enable a more intimate and immersive understanding of the issues that matter.
続きを読む »
 Alzheimer's drug that can slow disease gets backing from FDA advisersIf the agency agrees with its advisers, the drug would only be the second Alzheimer's drug approved to slow cognitive decline due to Alzheimer's.
Alzheimer's drug that can slow disease gets backing from FDA advisersIf the agency agrees with its advisers, the drug would only be the second Alzheimer's drug approved to slow cognitive decline due to Alzheimer's.
続きを読む »