Patients who are on the medication don’t need to stop taking it immediately, but are advised to discuss an alternative treatment with their healthcare provider.
Blood pressure medication Quinapril was voluntarily recalled last week over concerns it could increase the risk of cancer.Four lots of the blood pressure medication Quinapril were voluntarily recalled last week by drug manufacturer Lupin Pharmaceuticals Inc. over concerns it could increase the risk of cancer, Lupin Pharmaceuticals Inc.’s Quinapril tablets, sold in 20 mg and 40 mg, may contain an elevated level of nitrosamine impurity, N-Nitroso-Quinapril, above the acceptable daily intake level.
“Everyone is exposed to some level of nitrosamines,” the recall states. “ These impurities may increase the risk of cancer if people are exposed to them above acceptable levels over long periods of time.” The affected lots were distributed from March 2021 to Sept. 2022 and have expiry dates ranging from Dec. 2022 to March 2024. They came in 90-count bottles. See the
Lupin Pharmaceuticals Inc. said it stopped making the drug in September, and was working with distributors to arrange for the return of the recalled product lots. No illnesses associated with the recalled drug have been reported thus far, the company said.
日本 最新ニュース, 日本 見出し
Similar News:他のニュース ソースから収集した、これに似たニュース記事を読むこともできます。
 Blood pressure tablets recalled over potential cancer risk, FDA announcesFour lots of the blood pressure medication Quinapril were voluntarily recalled over concerns it could increase the risk of cancer, the Food and Drug Administration said.
Blood pressure tablets recalled over potential cancer risk, FDA announcesFour lots of the blood pressure medication Quinapril were voluntarily recalled over concerns it could increase the risk of cancer, the Food and Drug Administration said.
続きを読む »
 Blood pressure tablets recalled over potential cancer risk, FDA announcesThe FDA announced the second recall in months of blood-pressure medication over an elevated cancer risk. This time Quinapril tablets are being pulled.
Blood pressure tablets recalled over potential cancer risk, FDA announcesThe FDA announced the second recall in months of blood-pressure medication over an elevated cancer risk. This time Quinapril tablets are being pulled.
続きを読む »
 U.S. FDA weighs regulating cannabis compound CBD in food, supplements - WSJAfter weighing the evidence on the compound's safety, the FDA will decide within months how to regulate legal cannabis and whether that will require new agency rules or new legislation from Congress, according to the report. Cannabis products, excluding Jazz Pharmaceuticals PLC's Epidiolex, are illegal at the federal level in the United States, although some states allow their use. The agency wants to know if CBD can be safely eaten every day for a long period or during pregnancy amid concerns about future fertility, Patrick Cournoyer, who heads the FDA office developing the agency's cannabis strategy, told WSJ.
U.S. FDA weighs regulating cannabis compound CBD in food, supplements - WSJAfter weighing the evidence on the compound's safety, the FDA will decide within months how to regulate legal cannabis and whether that will require new agency rules or new legislation from Congress, according to the report. Cannabis products, excluding Jazz Pharmaceuticals PLC's Epidiolex, are illegal at the federal level in the United States, although some states allow their use. The agency wants to know if CBD can be safely eaten every day for a long period or during pregnancy amid concerns about future fertility, Patrick Cournoyer, who heads the FDA office developing the agency's cannabis strategy, told WSJ.
続きを読む »
 WSJ News Exclusive | FDA, Concerned About Safety, Explores Regulating CBD in Foods, SupplementsThe agency is studying whether cannabis-derived ingredients are safe in food and supplements and aims to reveal its oversight plans in the coming months.
WSJ News Exclusive | FDA, Concerned About Safety, Explores Regulating CBD in Foods, SupplementsThe agency is studying whether cannabis-derived ingredients are safe in food and supplements and aims to reveal its oversight plans in the coming months.
続きを読む »
 FDA Fast-Tracks Overdose Drug Naloxone for Use Without PrescriptionA pharmaceutical nonprofit was granted priority review from the FDA to make an inexpensive overdose-reversal drug for use without a prescription
FDA Fast-Tracks Overdose Drug Naloxone for Use Without PrescriptionA pharmaceutical nonprofit was granted priority review from the FDA to make an inexpensive overdose-reversal drug for use without a prescription
続きを読む »
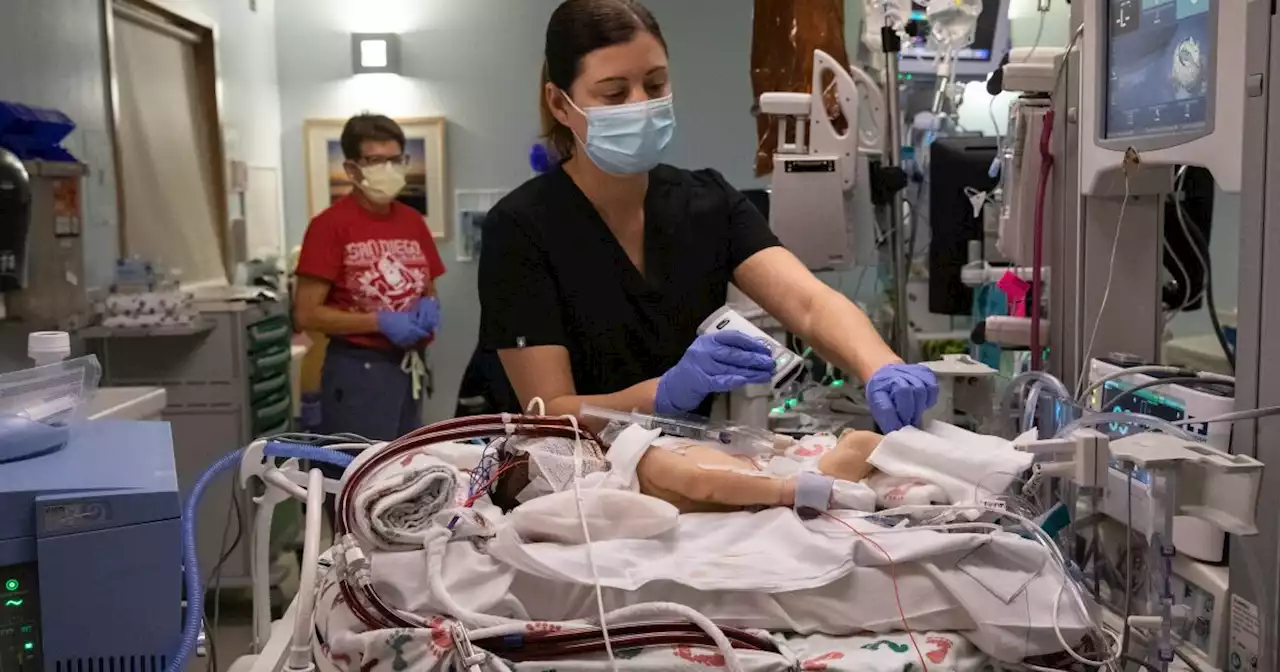 Region's newborns in crosshairs of possible blood shortageBabies who need transfusions often require O negative blood which is threatened by supply shortage
Region's newborns in crosshairs of possible blood shortageBabies who need transfusions often require O negative blood which is threatened by supply shortage
続きを読む »
