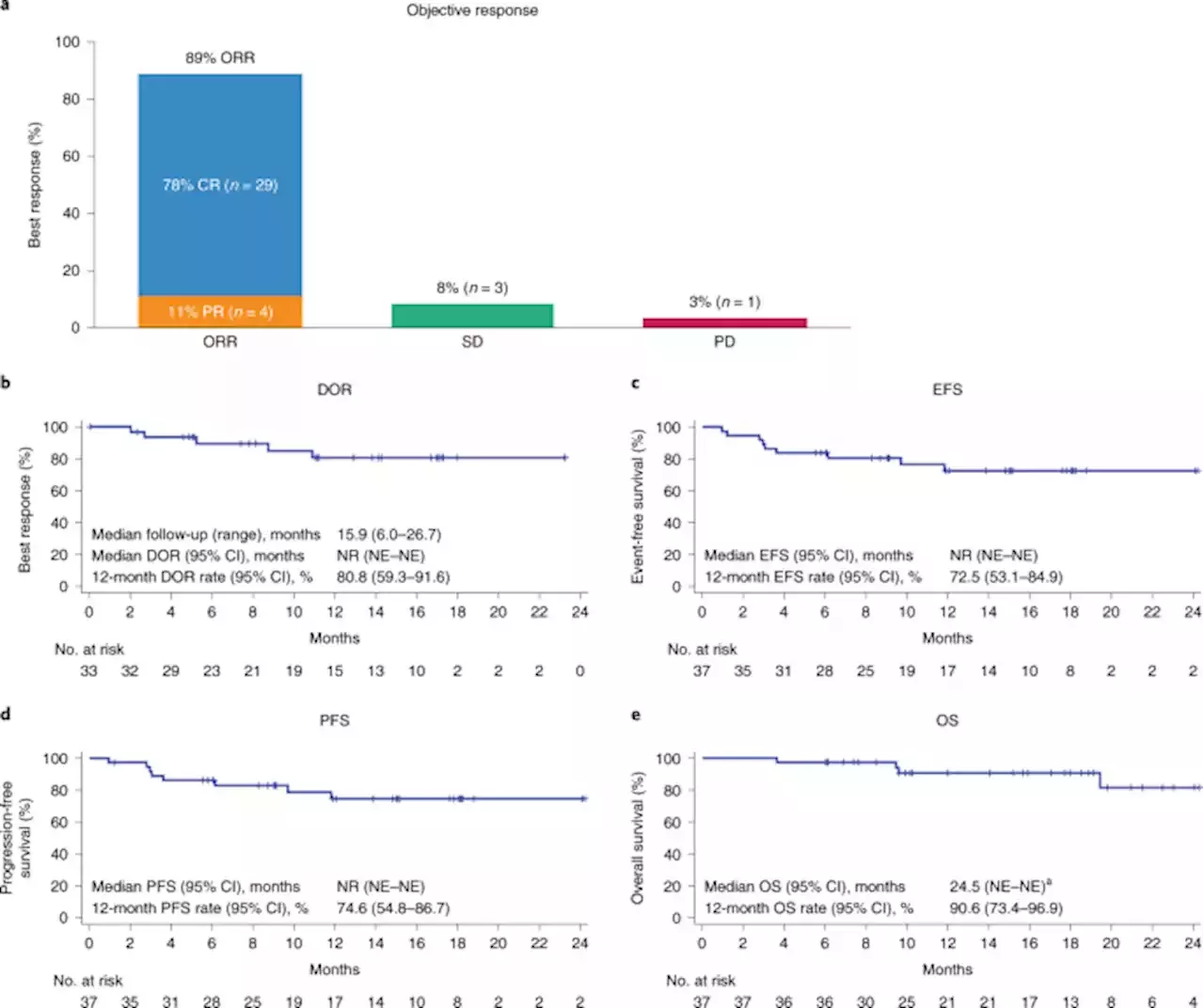
Presented at ASH21: In the phase 2 ZUMA-12 trial, first-line treatment with axi-cel CARTcells exhibited a high complete response rate and a manageable safety profile in adults with high-risk large B cell lymphoma MDAndersonNews MoffittNews ASH22
; serum alanine aminotransferase and aspartate aminotransferase ≤2.5 upper limit of normal; total bilirubin ≤1.5 mg dl, except in patients with Gilbert’s syndrome; cardiac ejection fraction ≥50%); no evidence of pericardial effusion as determined by echocardiogram, and no clinically notable electrocardiogram findings; no clinically notable pleural effusion; and baseline oxygen saturation >92% on room air.
Patients were excluded from the trial if any of the following applied: a history of malignancy other than nonmelanoma skin cancer or carcinoma in situ, unless disease-free for at least 3 years; a history of Richter’s transformation of chronic lymphocytic leukemia or PMBCL; a history of autologous or allogeneic stem cell transplantation; previous CD19-targeted therapy previous CAR T-cell therapy or other genetically modified T-cell therapy; history of severe, immediate hypersensitivity reaction...
All patients enrolled in the study provided written informed consent, and the study was conducted in accordance with applicable International Conference on Harmonisation Good Clinical Practice Guidelines, the principles of the Declaration of Helsinki and any applicable local laws and regulations. The protocol was approved by the institutional review board or independent ethics committee at each study site –Service Hematologic Seniors) and was provided to the key sponsor contact.
Within approximately 5 days of eligibility, laboratory monitoring began and then continued on days −5, −4 and −3; day 0; and days 1–7 after infusion. Post-treatment follow-up monitoring occurred at weeks 2 and 4, months 2 and 3 and then every 3 months thereafter until month 24. Disease response assessment was performed locally by PET–CT at week 4, month 3 and then every 3 months thereafter until month 24 or until disease progression, whichever occurred first.
All adverse events observed by the investigator or reported by the patient that occurred from enrollment up to 3 months after treatment with axi-cel infusion were reported. After 3 months, targeted adverse events were monitored and reported for 24 months after treatment with axi-cel or until disease progression, whichever occurred first. For patients who were enrolled but did not receive axi-cel, the adverse event-reporting period ended 30 days after the final study-specific procedure .
日本 最新ニュース, 日本 見出し
Similar News:他のニュース ソースから収集した、これに似たニュース記事を読むこともできます。
 Impact of the Human Cell Atlas on medicine - Nature MedicineIn their Perspective, Aviv Regev, Sarah Teichmann & colleagues discuss how cell atlases provide the missing links between genes, diseases and therapies — with benefits already seen in COVID-19, cancer and more teichlab sangerinstitute Cambridge_Uni
Impact of the Human Cell Atlas on medicine - Nature MedicineIn their Perspective, Aviv Regev, Sarah Teichmann & colleagues discuss how cell atlases provide the missing links between genes, diseases and therapies — with benefits already seen in COVID-19, cancer and more teichlab sangerinstitute Cambridge_Uni
続きを読む »
 Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial - Nature MedicineIn a prespecified interim analysis of a pivotal phase 2 trial, tisagenlecleucel, an autologous CD19-targeting CAR-T cell therapy, produced a high rate of complete responses with a manageable safety profile in adults with relapsed or refractory follicular lymphoma
Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial - Nature MedicineIn a prespecified interim analysis of a pivotal phase 2 trial, tisagenlecleucel, an autologous CD19-targeting CAR-T cell therapy, produced a high rate of complete responses with a manageable safety profile in adults with relapsed or refractory follicular lymphoma
続きを読む »
 Safety and efficacy of avapritinib in advanced systemic mastocytosis: the phase 1 EXPLORER trial - Nature MedicinePresented at ASH21: In the phase 1 EXPLORER trial, avapritinib, a selective KIT inhibitor, was generally well tolerated and elicited durable clinical and molecular responses in patients with advanced systemic mastocytosis MPNsm ASH22
Safety and efficacy of avapritinib in advanced systemic mastocytosis: the phase 1 EXPLORER trial - Nature MedicinePresented at ASH21: In the phase 1 EXPLORER trial, avapritinib, a selective KIT inhibitor, was generally well tolerated and elicited durable clinical and molecular responses in patients with advanced systemic mastocytosis MPNsm ASH22
続きを読む »
 Antigen-independent activation enhances the efficacy of 4-1BB-costimulated CD22 CAR T cells - Nature MedicineA bedside-to-bench analysis identifies single-chain variable fragment linker length as an important component of chimeric antigen receptor (CAR) structure and suggests that, in contrast to CD28-based CAR T cells, tonic signaling can be beneficial for 4-1BB-based CAR T cell function.
Antigen-independent activation enhances the efficacy of 4-1BB-costimulated CD22 CAR T cells - Nature MedicineA bedside-to-bench analysis identifies single-chain variable fragment linker length as an important component of chimeric antigen receptor (CAR) structure and suggests that, in contrast to CD28-based CAR T cells, tonic signaling can be beneficial for 4-1BB-based CAR T cell function.
続きを読む »
 Targeted therapy for osteoarthritis: progress and pitfalls - Nature MedicineNews and Views: Osteoarthritis is highly heterogeneous, so a 'one size fits all' approach to therapy is unlikely to succeed. Susanne Grässel & Nicole Schäfer discuss new trial data and the progress and pitfalls of targeted therapy for OA. uni_regensburg
Targeted therapy for osteoarthritis: progress and pitfalls - Nature MedicineNews and Views: Osteoarthritis is highly heterogeneous, so a 'one size fits all' approach to therapy is unlikely to succeed. Susanne Grässel & Nicole Schäfer discuss new trial data and the progress and pitfalls of targeted therapy for OA. uni_regensburg
続きを読む »
 Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy - Nature MedicineIn an analysis of adult patients with hematologic malignancies who received anti-CD19 chimeric antigen receptor T cell therapy, baseline gut microbiome composition was correlated with clinical response and treatment with broad-spectrum antibiotics in the four weeks prior to infusion was associated with worse survival and increased neurotoxicity.
Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy - Nature MedicineIn an analysis of adult patients with hematologic malignancies who received anti-CD19 chimeric antigen receptor T cell therapy, baseline gut microbiome composition was correlated with clinical response and treatment with broad-spectrum antibiotics in the four weeks prior to infusion was associated with worse survival and increased neurotoxicity.
続きを読む »