Alnylam Pharmaceuticals Inc on Thursday filed lawsuits in Delaware federal court against Pfizer Inc and Moderna Inc , claiming their multibillion-dollar mRNA COVID-19 vaccines infringe one its patents.
The U.S. National Institutes of Health has also implied it may sue Moderna over a vaccine-related patent application that NIH says should have listed its scientists as co-inventors.
Alnylam asked the court for an undisclosed amount of money damages from Pfizer and Moderna. It said in a statement it does not intend to take action that impedes the production, sale or distribution of the vaccines. Moderna has said its vaccine earned the company $17.7 billion in revenue in 2021. Pfizer said last month that it expected $32 billion in revenue from its vaccine this year.Reporting by Manojna Maddipatla in Bengaluru and Blake Brittain in Washington, D.C.; Editing by David Bario and Bill BerkrotSign up to our legal newsletter for a smart look at the day's headlines concerning the practice of law.
日本 最新ニュース, 日本 見出し
Similar News:他のニュース ソースから収集した、これに似たニュース記事を読むこともできます。
 AP Source: Pfizer Seeking OK for 4th COVID Dose for SeniorsThe move would add a fourth dose to the COVID vaccine regimen, which currently consists of a primary series of two shots, followed months later by a booster dose, in an effort to provide maximum protection to the over-65 population that has been hit hardest by the pandemic.
AP Source: Pfizer Seeking OK for 4th COVID Dose for SeniorsThe move would add a fourth dose to the COVID vaccine regimen, which currently consists of a primary series of two shots, followed months later by a booster dose, in an effort to provide maximum protection to the over-65 population that has been hit hardest by the pandemic.
続きを読む »
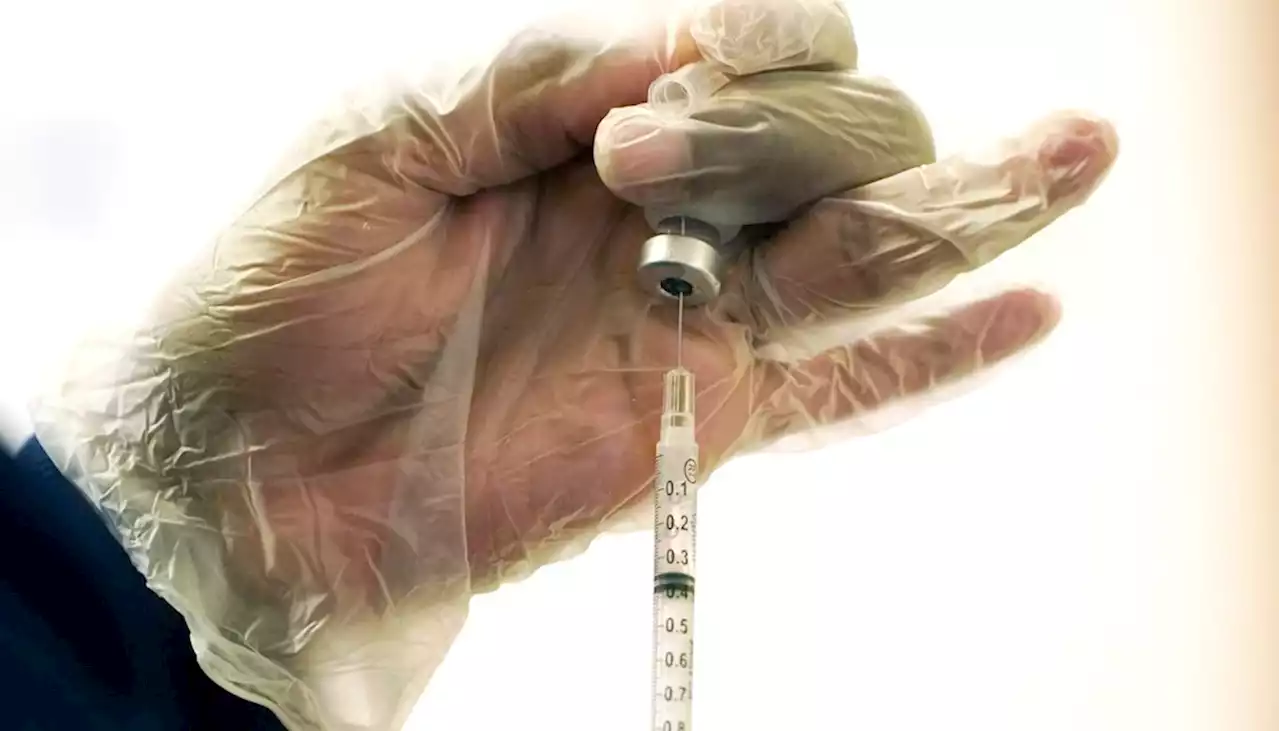 AP source: Pfizer seeking OK for 4th COVID dose for seniorsThe Food and Drug Administration and the Centers for Disease Control must approve the request.
AP source: Pfizer seeking OK for 4th COVID dose for seniorsThe Food and Drug Administration and the Centers for Disease Control must approve the request.
続きを読む »
 Pfizer seeks approval for 4th COVID-19 booster dose for seniorsPfizer is expected to request authorization for a fourth COVID-19 vaccine dose for seniors in a move to provide extra protection to the over-65 population that has been hit hardest by the pandemic.
Pfizer seeks approval for 4th COVID-19 booster dose for seniorsPfizer is expected to request authorization for a fourth COVID-19 vaccine dose for seniors in a move to provide extra protection to the over-65 population that has been hit hardest by the pandemic.
続きを読む »
 Pfizer to seek authorization for 2nd Covid booster for people 65 and olderIf the FDA authorizes it, the additional shot would go to people who are among those with the highest risk of serious illness.
Pfizer to seek authorization for 2nd Covid booster for people 65 and olderIf the FDA authorizes it, the additional shot would go to people who are among those with the highest risk of serious illness.
続きを読む »
 Pfizer/BioNTech seek FDA authorization for fourth Covid-19 vaccine doses for people 65 and upPfizer and BioNTech submitted an application for emergency use authorization to the US Food and Drug Administration for an additional booster dose of their Covid-19 vaccine for adults 65 and older who have gotten a booster dose of any of the authorized or approved vaccine, the companies said Tuesday.
Pfizer/BioNTech seek FDA authorization for fourth Covid-19 vaccine doses for people 65 and upPfizer and BioNTech submitted an application for emergency use authorization to the US Food and Drug Administration for an additional booster dose of their Covid-19 vaccine for adults 65 and older who have gotten a booster dose of any of the authorized or approved vaccine, the companies said Tuesday.
続きを読む »
 Pfizer seeks authorization for 2nd COVID-19 booster for older adultsPfizer's CEO confirmed on Twitter that they seek emergency use authorization to provide a second COVID-19 booster shot for adults 65 years and older.
Pfizer seeks authorization for 2nd COVID-19 booster for older adultsPfizer's CEO confirmed on Twitter that they seek emergency use authorization to provide a second COVID-19 booster shot for adults 65 years and older.
続きを読む »
