The oysters were reportedly harvested and sold by Future Seafoods, Inc. in Prince Edward Island, Canada to U.S. importers in six states.
Restaurants and retailers in half a dozen states have been advised by the Food and Drug Administration to dispose of oysters purchased from a company based in Canada due to possible salmonella and E. coli contamination.
The Canadian Food Inspection Agency tested the oysters and discovered the presence of salmonella and E. coli, and then reportedly informed the FDA on Oct. 18. The contaminated oysters could cause illness if eaten raw. The FDA said food that contains salmonella and E. coli may look, smell and taste normal.
日本 最新ニュース, 日本 見出し
Similar News:他のニュース ソースから収集した、これに似たニュース記事を読むこともできます。
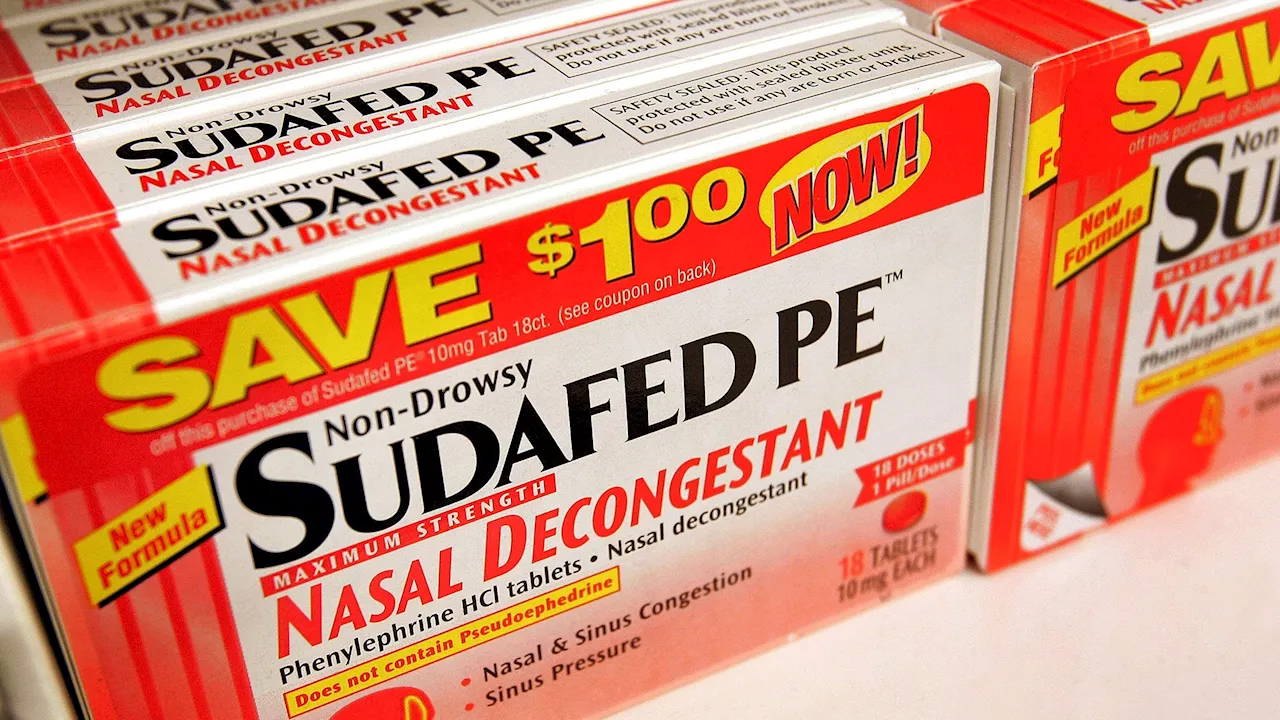 CVS to stop selling cold and allergy pills that FDA warns don’t workLaura is a science news writer, covering a wide variety of subjects, but she is particularly fascinated by all things aquatic, paleontology, nanotechnology, and exploring how science influences daily life. Laura is a proud former resident of the New Jersey shore, a competitive swimmer, and a fierce defender of the Oxford comma.
CVS to stop selling cold and allergy pills that FDA warns don’t workLaura is a science news writer, covering a wide variety of subjects, but she is particularly fascinated by all things aquatic, paleontology, nanotechnology, and exploring how science influences daily life. Laura is a proud former resident of the New Jersey shore, a competitive swimmer, and a fierce defender of the Oxford comma.
続きを読む »
 FDA Warns Pet Owners After Dog Food Recalled Over Salmonella RiskThe administration has offered advice and guidance to anyone concerned that they may have been impacted by the recalled product.
FDA Warns Pet Owners After Dog Food Recalled Over Salmonella RiskThe administration has offered advice and guidance to anyone concerned that they may have been impacted by the recalled product.
続きを読む »
 FDA Warns of Hidden Ingredients in Arthritis, Pain ProductsCertain products may contain active ingredients found in prescription-only drugs.
FDA Warns of Hidden Ingredients in Arthritis, Pain ProductsCertain products may contain active ingredients found in prescription-only drugs.
続きを読む »
 FDA proposes ban of toxic chemical found in hair products used primarily by Black womenBeauty can be a pain and in some cases it can even be cancerous. Hair relaxers are most commonly used on African American women's hair to chemically straighten
FDA proposes ban of toxic chemical found in hair products used primarily by Black womenBeauty can be a pain and in some cases it can even be cancerous. Hair relaxers are most commonly used on African American women's hair to chemically straighten
続きを読む »
 AstraZeneca said U.S. FDA has accepted for review its Flumist self-administered flu vaccineCiara Linnane is MarketWatch's investing- and corporate-news editor. She is based in New York.
AstraZeneca said U.S. FDA has accepted for review its Flumist self-administered flu vaccineCiara Linnane is MarketWatch's investing- and corporate-news editor. She is based in New York.
続きを読む »
FDA to Review First Self-Administered Flu VaccineAstraZeneca said on Tuesday the U.S. Food and Drug Administration (FDA) has accepted for review the company's application seeking approval for patients or caregivers to administer its nasal flu vaccine. If approved, the vaccine - branded as FluMist Quadrivalent - could...
続きを読む »
