The FDA says it is waiting for data to be submitted next year to clear shots for some of the youngest Pfizer vaccine recipients.
, following a decision to expand emergency use authorization for the shots from Moderna and Pfizer-BioNTech to most children as young as 6 months old.on Thursday, clearing all recipients of Moderna's COVID-19 vaccine to get a booster dose from the company's newest"bivalent" vaccines — which are tailored to recent Omicron variants — at least two months after their last"monovalent" shot.
Now the FDA says only kids who have yet to complete their third dose can swap out the last shot for an updated vaccine. Early adopters who already completed their third shot some several months ago must wait until the FDA receives more data next year. "At this time, we do not have clinical data in this age group for a fourth dose of the original vaccine following the third primary series dose, and thus we cannot yet extrapolate," Pfizer spokesperson Julia Michelle Cohen said in an email.
日本 最新ニュース, 日本 見出し
Similar News:他のニュース ソースから収集した、これに似たニュース記事を読むこともできます。
 Pfizer partners with Clear Creek Bio to develop oral COVID-19 drugPfizer Inc and Clear Creek Bio Inc on Tuesday announced a collaboration to identify a potential drug candidate and develop a new class of oral treatment against COVID-19, as Pfizer seeks to expand its anti-infective pipeline.
Pfizer partners with Clear Creek Bio to develop oral COVID-19 drugPfizer Inc and Clear Creek Bio Inc on Tuesday announced a collaboration to identify a potential drug candidate and develop a new class of oral treatment against COVID-19, as Pfizer seeks to expand its anti-infective pipeline.
続きを読む »
 FDA authorizes updated COVID shots for kids as young as 6 months oldThe FDA is encouraging families to get young children vaccinated ahead of the holidays.
FDA authorizes updated COVID shots for kids as young as 6 months oldThe FDA is encouraging families to get young children vaccinated ahead of the holidays.
続きを読む »
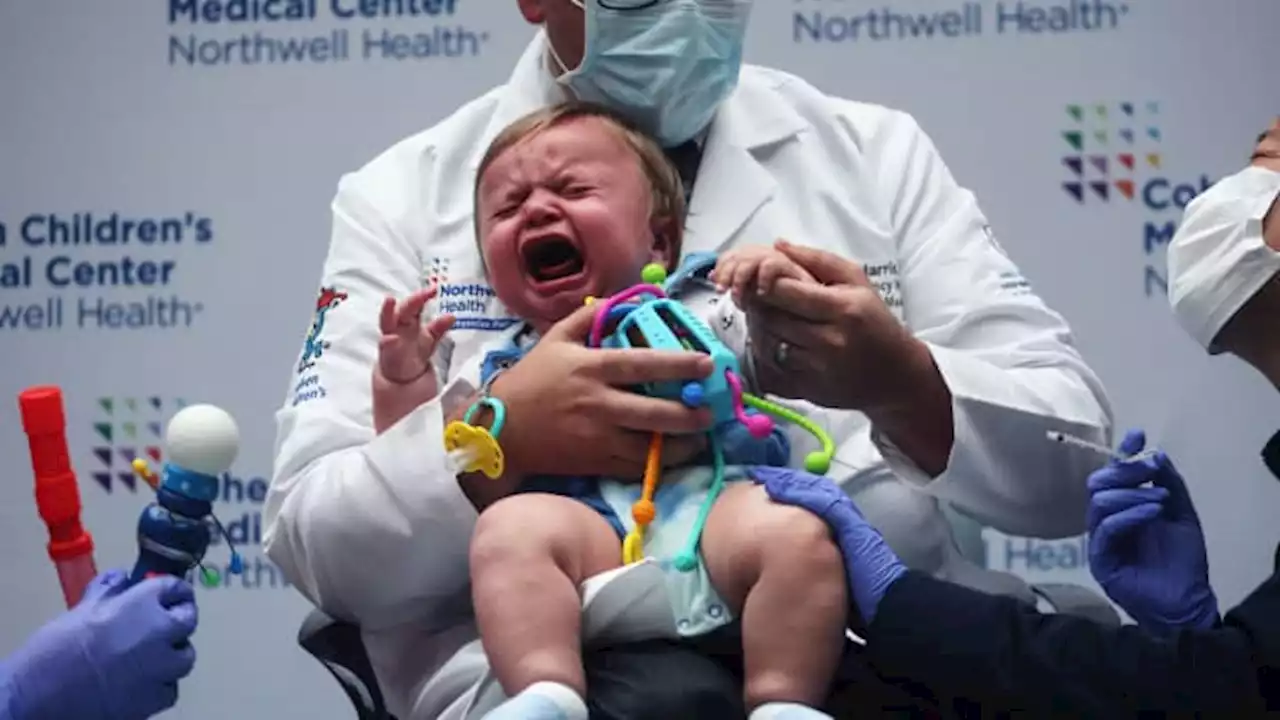 FDA authorizes Covid omicron vaccines for children as young as 6 months old
FDA authorizes Covid omicron vaccines for children as young as 6 months old
続きを読む »
 FDA clears updated COVID-19 vaccines for kids under age 5U.S. regulators have cleared doses of the updated COVID-19 vaccines to children younger than 5.
FDA clears updated COVID-19 vaccines for kids under age 5U.S. regulators have cleared doses of the updated COVID-19 vaccines to children younger than 5.
続きを読む »
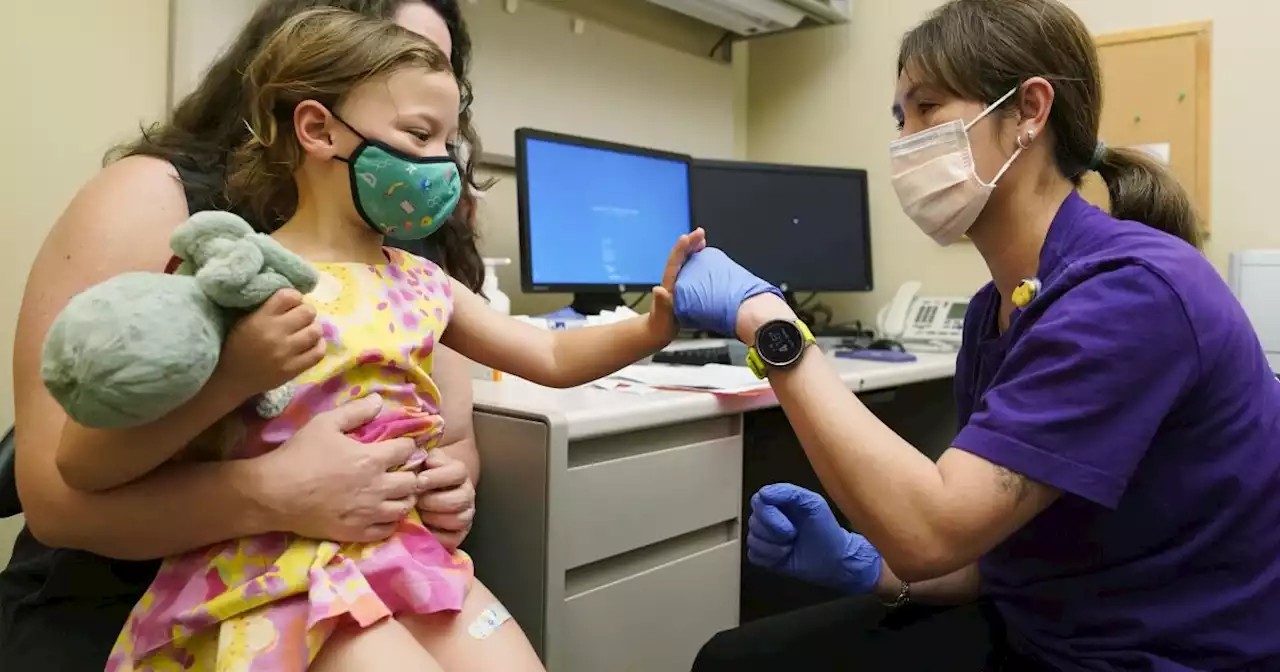 FDA clears updated COVID-19 vaccines for kids under age 5U.S. regulators have cleared pediatric doses of the updated COVID-19 vaccines to children ages 6 month to 5 years.
FDA clears updated COVID-19 vaccines for kids under age 5U.S. regulators have cleared pediatric doses of the updated COVID-19 vaccines to children ages 6 month to 5 years.
続きを読む »
